Abstract
Microcephalic children due congenital Zika virus syndrome (CZS) present neurological symptoms already well described. However, several other alterations can also be observed. Here, we aimed to evaluate the immune system of microcephaly CZS children. We showed that these patients have enlarged thymus, spleen and cervical lymph nodes, analysed by ultrasound and compared to the reference values for healthy children. In the periphery, they have an increase in eosinophil count and morphological alterations as hypersegmented neutrophils and atypical lymphocytes, even in the absence of urinary tract infections, parasitological infections or other current symptomatic infections. Microcephalic children due CZS also have high levels of IFN-γ, IL-2, IL-4, IL-5 and type I IFNs, compared to healthy controls. In addition, this population showed a deficient cellular immune memory as demonstrated by the low reactivity to the tuberculin skin test even though they had been vaccinated with BCG less than 2 years before the challenge with the PPD. Together, our data demonstrate for the first time that CZS can cause alterations in primary and secondary lymphoid organs and also alters the morphology and functionality of the immune system cells, which broadens the spectrum of CZS symptoms. This knowledge may assist the development of specific therapeutic and more efficient vaccination schemes for this population of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zika Virus (ZIKV) is an emergent flavivirus that was first isolated in 1947 in Uganda [1] and in 2015 its arrival was confirmed in Brazil [2, 3]. The classic symptoms of the disease are mild, such as low fever, non-purulent conjunctivitis, retro-ocular pain, maculopapular rash, headache, myalgia, gastrointestinal disorders and arthralgia. However, the ZIKV epidemic was also associated with malformations in the central nervous system (CNS) of fetuses after the detection of the virus in the blood, amniotic fluid and nervous tissue of embryos after congenital ZIKV infection [4, 5].
Children with congenital zika virus syndrome (CZS) may have symptoms as microcephaly, occipital bone protuberance, excess and/or skin folds in the scalp, umbilical hernia, severe global hypertonia, irritability, hyperexcitability, impaired auditory and visual responses, epileptic spasms, arthrogryposis, retinal and optic nerve changes, etc. [6]. Among the whole range of symptoms, microcephaly, which is generally severe, draws attention due to the craniofacial disproportion and the serious consequences on the child's neurodevelopment. Microcephaly is characterized by a malformation in the CNS in which head circumference is at least 2 standard deviations (SD) below the average for gestational age and gender. It usually causes delays in neurological, mental and motor development [7,8,9] and has no cure. Due to the high tropism of the virus by almost all tissues, many other aspects of the CZS are being discovered after monitoring the first generation of children affected, such as obesity caused by endocrine disorders, heart problems, susceptibility to infections in the airways and urinary tract [10] and high concentration of inflammatory peripheral markers [11]. Taking into account the range of symptoms presented by CZS children, it is possible that in utero exposure compromises the formation and development of several organs, not just the CNS.
Immune system development begins during the intrauterine period and is completed at approximately 10 years of age [12]. The thymus is the first lymphoid organ that begins to develop, which occurs around the 2nd week of gestation [13, 14]. Lymphoid and myeloid progenitors are found in the yolk sac since the 4th week [12], and lymph nodes development starts at the 5th week [15]. At week 6, the development of the spleen begins [16], and at week 7, lymphoid and myeloid progenitors migrate to the liver, where they proliferate and undergo a slight differentiation [12]. Then, the T lymphocyte precursors migrate to the thymus, where its maturation is concluded still in the intrauterine period [12]. During embryogenesis, the number of lymphocytes in the fetal circulation increases linearly with gestational age [17]. Under normal conditions, at birth, the levels of T lymphocytes are high, and they increase even more in the first year; then, they decrease and normalize to adult levels up to 2 years of age [18, 19]. The cytokine production is polarized to the Th2 profile up to one year of age [20], probably due to the low production of IL-12 by the newborn [21]. Under normal conditions, the spleen, thymus and cervical lymph nodes have their development completed after birth and are functional [14,15,16].
Ideally, the organogenesis process should not be affected by congenital infections that cause inflammatory reactions because it predisposes damage to various organs and systems. It has been observed that children who had intrauterine infections by Epstein-Barr virus or cytomegalovirus maintain the Th2 profile until at least 2 years of age, which can increase the probability of developing allergies [22] and susceptibility to intracellular pathogens infections. In addition, newborns with congenital toxoplasmosis showed an increase in monocytes, NK cells, CD4+ T cells and CD8+ T cells with a proinflammatory profile [23].
The peculiarities of the immune system of children with microcephaly due CZS are still poorly understood so far, and as ZIKV congenital infection occurs during a critical period for lymphoid organs formation, it may compromise the children’s immune system and lead to different levels of immunosuppression. Here, we showed that microcephalic children due CZS have morphometric alteration in lymphoid organs, hematological and cytokine imbalance with impaired generation of cellular immune memory to the Bacillus Calmette-Guérin (BCG) vaccine. These discoveries may provide clues to understand the extension of the symptoms of CZS and also contribute to guiding the choice of most efficient therapeutic and vaccination schedules for those patients.
Materials and methods
Study population
The subjects (n = 42) were children aged from 9 to 65 months old, both genders. They were divided into two groups: the control group (n = 20 healthy children without congenital syndromes, microcephaly, neuromotor disorders, symptomatologic infections and negative parasitological assay; 50% male and 50% female, average age of 35.8 months, SD = 19.2), and the microcephaly group (n = 22 children diagnosed at birth, 56.5% male and 43.5% female, average age of 33.5 months (SD = 11.7) (Table 1). The CZS diagnosis was performed according to the Brazilian Ministry of Health [24] and as previously described [25,26,27]. All the patients were negative for ZIKV in the blood, tested following the protocol described by Faye et al. [28].
All children were recruited at the Anita Garibaldi Center for Education and Research in Health (CEPS, Macaíba-RN). Children with secondary immunodeficiency or autoimmune disorders, children who were vaccinated or suspected of being infected in the past 14 days and children who had a positive parasitological test and/or urine culture examination were excluded from the study. The study was performed according to human experimental guidelines of the Brazilian Ministry of Health and the Helsinki Declaration. The project was approved by the Committee of Ethics in Research with Human Beings (CAAE 74871317.8.0000.5292 and 17583419.7.0000.5537). The data and sample collections were started after completing and signing the informed consent form by the children's legal guardians, who also answered a clinical questionnaire. The experimental design of the study is shown in Fig. 1.
Study design. Forty-two children between 9 and 65 months of age, of both genders, were recruited for the study. The children were divided into the following two groups: a control group composed of 20 healthy children and a microcephaly group composed of 22 children diagnosed with CZS. The control group was recruited just once and the microcephalic group was recruited four times, as indicated by the grayscale colours. All microcephalic children’s legal guardians answered the clinical questionnaire. The first exam performed was ultrasonography (USG) of the spleen, thymus and cervical lymph nodes in 21 children with microcephaly. One of the children was lost in the follow-up due to non-release of the medical report. Subsequently, the first blood collection in 13 children with microcephaly was used to perform automated complete blood counts. The urine culture exam was carried out with eight microcephalic children. The second blood collection was performed with twenty children with microcephaly to perform the CBA, the differential cell countings, qRT-PCR for type I IFN, C-reactive protein and rheumatoid factor measurements. On the same day, fecal samples for parasitological assays of 15 children were collected. The tuberculin test was performed in 7 microcephalic children. A total of 15 patients were lost during the follow-up. The 20 children in the control group were recruited with their respective legal guardians only for the third set of tests. Blood and faecal samples were collected on the same day. Four control children had a positive parasitological examination and were excluded from the study, thus, the application of the clinical questionnaire, the CBA, the differential cell countings, qRT-PCR and rheumatoid factor measurement were performed in 16 control children, C-reactive protein measurement was performed in 12 children. A total of four individuals from the control group were lost during the follow-up. “Unavailable sample” refers to an impossibility of sample collection in that set of experiments or insufficient sample for all the tests, but the patient was not excluded from the study
Morphometric evaluation of the spleen, thymus and cervical lymph nodes by ultrasound
Morphometric evaluation was performed once with 21 children with microcephaly. The Medisonic Accuvix A30 LV, DMR + 2.0, Sonoview II, 3D/4D (Samsung) ultrasound device with convex (PB-AKC2-6IC) and/or linear (PB-AKL5-13IS) transducer was used to assess images from the spleen, thymus and cervical lymph nodes of microcephalic children by a specialist physician. All mappings were performed in B-mode. To evaluate the thymus, the suprasternal, parasternal or sternal accesses were used to analyse the contour, echotexture, topography, longitudinal and antero-posterior diameters. The spleen was assessed through the upper abdominal region and the volume analysed. Cervical lymph nodes were assessed for diameter, morphology and echotexture evaluation. The patients were grouped by age for the analysis of the lymphoid organs measurements, so the stage of child development could be considered. For cases in which the ethics committee did not allow collection in the control group, data were compared with reference values for healthy individuals paired by gender and age [29,30,31,32] added in the figures as dotted squares.
Blood collection, complete blood count and differential leukocyte count
The peripheral blood samples from patients from both groups were collected by venipuncture with a sterile disposable syringe in a tube containing ethylenediamine tetraacetic acid (EDTA). Blood collection was performed at two different time points. The first one was performed with 13 children with microcephaly for automated complete blood count on the same day that the ultrasound examinations were performed. In the second blood collection, 16 children of the control group and 15 microcephalic children had the blood collected. On the same day, two faecal samples of each child were also collected for parasitological assays. The time between the first and the second blood collection was a maximum of 7 months. From the second blood collection, the serum was used for the CBA experiment and a blood smear for differential leukocyte counting, stained by the rapid panoptic method. To search for morphological alterations, specific counts were performed in 100 lymphocytes, 100 neutrophils and 10 eosinophil cell populations per slide. All counts were performed using a 100x objective in an optical microscope with immersion oil, read in the tail region. Photomicrographs were performed using the ZEISS Axio Imager 2 microscope coupled with AxioCam Mrc 5 camera using the 63x objective. The software used for image acquisition was Neurolucida 11 from MBF Bioscience (Vermont, EUA). For analyses for which the ethics committee did not allow collection in the control group, reference values of the laboratory that performed the automated paediatric blood counts and that were released in the reports were used for analysis and comparison added in the figures as dotted squares.
Cytokine measurement
The cytokines IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-10 and TNF-α were quantified in serum samples from 16 children of the control group and 15 microcephalic children by the Cytometric Bead Array (CBA, Flex Cytometric Bead Array Enhanced Sensitivity, BD Pharmingen, USA), according to the manufacturer's instructions. The limit of detection for each cytokine was the following: 14,84 fg/mL for IFN-γ, 88,9 fg/mL for IL-2, 144,4 fg/mL for IL-4, 67,8 fg/mL for IL-5, 68,4 fg/mL for IL-6, 13,7 fg/mL for IL-10 and 67,3 fg/mL for TNF-α. The samples were acquired on the LSR Fortessa flow cytometer (BD Biosciences, USA), and data analysis was performed using the FCAP Array software (BD Biosciences, USA).
Total mRNA extraction, cDNA synthesis and qRT-PCR
Evaluation of IFN-α and IFN-β transcripts in whole blood samples was performed by real-time PCR. Total RNA was extracted using an SV Total RNA Isolation System Kit (Promega, CA, USA), and reverse transcription was performed with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA). PCR was performed using an Applied Biosystems 7500 system with specific primers and SYBR Green (Applied Biosystems, CA, USA) fluorescence detection reagents. GAPDH and β-Actin mRNA levels were used to normalize the mRNA content in all samples. Normalized expression was calculated as previously described by Livak, 2001 [33]. The Primers were only accepted if their efficiency was 100 ± 10%, their sequences are shown in Table 2. Corrections were made for primer efficiency. The specificity of the reaction was examined by the dissociation curve.
C-reactive protein and rheumatoid factor measurements
Qualitative tests for serum C-reactive Protein (CRP) and Rheumatoid Factor (RF) were performed by latex agglutination with the PCR—Látex Kit (Ebram, SP, Brazil) and Reumalátex Kit (Labtest, MG, Brazil), respectively, according to the manufacturer’s instructions.
Parasitological stool sample exam
Nondiarrheal faecal material was collected from 16 children of the control group and 15 microcephalic children. The sample was divided into two aliquots for each patient: one freshly stored in sterile collectors, and the other sample was stored in 20 mL of 10% formalin. The Hoffman, Pons and Janer (HPJ) and Baermann–Moraes methods were performed to search for helminth eggs, protozoan cysts and adult parasites.
Urine culture exam
To investigate urinary tract infections (UTIs), urine samples from 8 children with microcephaly were obtained by a vesical catheter for uroculture. The samples were collected once, up to 1 and 9 days after the first blood collection. The presence of UTI was characterized by bacterial growth greater than or equal to 100,000 CFU/mL, as previously described [34]. All children examined had a negative result for this examination.
Tuberculin test
The tuberculin skin test was performed in seven children with microcephaly who were vaccinated with the BCG vaccine up to 2 years before the tuberculin skin test. The PPD antigen (Brazilian Ministry of Health—prohibited trade, 2 UT/0.1 mL) was applied by intradermal injection, in the dose of 0.1 ml (0.04 mcg), on the anterior side of the left forearm, at an angle of 5–15 degrees, avoiding inoculation in areas with lesions, superficial veins or scars. After 72 h the reading was performed using a specific millimetre ruler, measuring the largest transverse diameter of the hardening perpendicularly to the forearm.
Data analysis and statistics
Statistical analyses were performed using PRISM® 6.0 software (GraphPad, San Diego, CA, USA). Agostino–Pearson and Shapiro–Wilk tests were performed to verify the Gaussian distribution. The Mann–Whitney test and t test were used to compare nonparametric and parametric data, respectively. To analyse nominal, categorical, nonparametric and independent data with expected frequency less than 5, Fisher test was used. p ≤ 0.05 was considered statistically significant.
Results
Children with microcephaly due CZS present morphometric alterations of the thymus, spleen and cervical lymph nodes
To understand the clinical history of the patients, first, the legal guardians answered a clinical questionnaire. Among the 22 microcephalic children, 31.8% (n = 7) had already been diagnosed with pneumonia (M = 2.12, SD = 2), 22.7% (n = 5) were hospitalized at least once in 2018 (M = 2, SD = 2) and 27.2% (n = 6) in 2019 (M = 1.28, SD = 0.48), with 50% (n = 3) of children hospitalized in 2019 being the same ones who were hospitalized in 2018, regardless of the cause (Table 1). Analysing the clinical history of the 16 non-microcephalic control children, only one child (6.25%) was diagnosed with pneumonia once since birth (M = 0.06, SD = 0.25), two children (12.5%) were hospitalized in 2018 (M = 0.12, SD = 0.34) and only one (6.25%) was hospitalized in 2019 (M = 0.12, SD = 0.5) (Table 1).
To initiate the characterization of the immune system in microcephalic children, we analysed the morphological and metric aspects of the lymphoid organs as thymus, spleen and cervical lymph nodes of 21 children with microcephaly by ultrasonography. All patients presented a regular thymus contour, 20 children (95.2%) presented a normal thymus echotexture, characterized as finely heterogeneous and hypoechoic, but one patient showed a coarse and diffusely heterogeneous echotexture of the thymus (Fig. 2A). Regarding the thymus topography, 81% (n = 17) had a retrosternal topography superior to the level of the wishbone, which indicates an organ increase (Fig. 2B). To analyse the longitudinal measurement of the thymus, the children were divided into the following three groups according to their ages: 8–10 months, 12–18 months and 24–96 months. All groups analysed presented the longitudinal measure of the thymus above the reference values for their ages (8–10 months: M = 6.1, SD = 1.27; 12–18 months: M = 6.75, SD = 2.47 and 24–96 months: M = 5.91, SD = 1.51) (Fig. 2C, G).
Microcephalic children due CZS show alterations in the thymus, spleen and cervical lymph nodes. Twenty-one microcephalic children due CZS were recruited for ultrasonography analysis of the lymphoid organs. We assessed the thymus echotexture (A), topography (B) and longitudinal measurement in centimetres (C and G); the spleen echotexture (D and H) and volume (E and H); and the maximum diameter of the cervical lymph nodes (F and I). T1, thymic longitudinal measurement in centimetres. S1, splenic longitudinal measurement; S2, transversal measurement; S3, anteroposterior measurement; Vol., volume. L1, lymph node maximum diameter. The graphs are plotted as the mean ± SD. The dotted squares correspond to the reference values set in the literature for healthy children without microcephaly paired by gender and age
Most of the children (90.4%, n = 19) presented a regular homogeneous echotexture of the spleen; however, two children showed alterations in the echotexture, one of them presented homogeneous echotexture with a small cystic and anechoic image and another presented homogeneous echotexture with some echogenic points (Fig. 2D). To analyse the splenic volume, the children were divided into the following two groups according to their ages: 9–48 and 48–60 months of age. The spleen volume of 52.36% (n = 11) of children from 9 to 48 months was above the reference value (M = 62.2, SD = 25.7) (Fig. 2E, H). However, the two children from 48 to 60 months presented regular volume within the reference value (M = 56.9, SD = 2.3) (Fig. 2E).
The last lymphoid organ evaluated was the lymph nodes from the right and left cervical chains because of their proximity to the brain. Comparing the maximum diameters (corresponding to the diameter of the largest cervical lymph node found during the examination) of the cervical lymph node from microcephalic children with the reference values, it is possible to observe that 17 patients (80.9%) had an increased maximum diameter (Fig. 2F, I). The lymph node morphology and echotexture were normal in all children analysed (data not shown). Together, the ultrasound image data from lymphoid organs showed alterations in thymus size and topography, spleen volume and changes in cervical lymph nodes’ diameter in the majority of the microcephalic children analysed here. It is also important to highlight the presence of fibrosis points in the thymus of one patient, echogenic points in the spleen of one child and a cyst of 0.4 cm in the spleen of another patient.
Children with microcephaly due CZS present morphological alterations in lymphocytes and neutrophils
In an attempt to evaluate whether the lymphoid organs alterations were accompanied by changes in immune cell populations on the periphery, we performed an automated complete blood count in 13 microcephalic children. It was observed that 46.14% (n = 6) of children with microcephaly had an increase in the number of total leukocytes (M = 10,286.2, SD = 3735.6) (Fig. 3A), 61.52% (n = 8) had an increase in eosinophils count (M = 610.64, SD = 520) (Fig. 3B), 23% (n = 3) of children had an increase in the number of lymphocytes (M = 5205.77, SD = 2221.9) (Fig. 3C), 15.38% (n = 2) of children had an increase in the number of segmented neutrophils (M = 3898.08, SD = 1762.2) (Fig. 3D) and 23% (n = 3) had an increase in the number of monocytes (M = 535.7, SD = 274.2) (Fig. 3E) compared to the reference values for healthy children without microcephaly paired by gender and age.
Microcephalic children with CZS have an increase in eosinophils count. Thirteen children with microcephaly had their blood collected for automated complete blood count. Total leukocytes (A), eosinophils (B), lymphocytes (C), segmented neutrophils (D) and monocytes (E) per mm3 were analysed. The graph is plotted as the mean ± SD. The dotted squares correspond to the reference values set in the literature for healthy children without microcephaly paired by sex and age
To investigate the peripheral blood cell morphology, we performed the differential leukocyte counts and specific counts of lymphocytes, neutrophils and eosinophils in 11 children with microcephaly and 16 healthy children. Microcephalic children showed a decrease in lymphocytes with normal morphology (p = 0.0044) (Fig. 4A, B) and an increase in lymphocytes with atypical morphology (p = 0.0044) (Fig. 4C, D), compared to the control group. Children with microcephaly also showed a significant reduction on neutrophils with normal morphology (p = < 0.0001) (Fig. 5A, B), higher numbers of hypersegmented neutrophils (p = < 0.0001) (Fig. 5C, D) and an increase in the counts of hypersegmentation (HS) + cytoplasmic vacuolation (VC) in the same neutrophil (p = 0.0188) (Fig. 5E, F) compared to healthy children. Morphological alterations such as cytoplasmic vacuolation (Fig. 5G, H), toxic granulation (TC) (Fig. 5I, J) and hypersegmentation + toxic granulation in the same cell (Fig. 5K, L) were also investigated in neutrophils, and there was no significant difference between microcephalic children and the control group. Eosinophils morphological alterations (hypersegmentation, cytoplasmic vacuolation and hypersegmentation + cytoplasmic vacuolation in the same cell) were similar in both groups (data not shown). As the alterations in number and cell morphology are compatible with current infectious processes, we also collected urine and faeces from CZS children to evaluate the presence of asymptomatic bacterial infections in the urinary tract or parasitological infections in the digestive tract, but all patients had negative results (data not shown). Four healthy controls had a positive parasitological examination and were excluded from the study.
Children with microcephaly due CZS have a decrease in the normal lymphocyte population and an increased number of a typical lymphocytes. Blood smears from 11 children with microcephaly and 16 healthy children without microcephaly were used for differential lymphocyte counts. Normal (A and B) and atypical (C and D) lymphocytes were analysed. The Mann–Whitney test was used in the analysis. The graphs are plotted as the mean ± SD. **p = 0.0044
CZS-microcephalic children have a decreased number of normal neutrophils and an increased number of hypersegmented and hypersegmented + vacuolated neutrophils. Blood smears from 11 children with microcephaly and 16 healthy control children without microcephaly were used for differential neutrophil counts. Normal (A and B), hypersegmented (HS) (C and D); hypersegmented and vacuolated (HS + VC) (E and F); vacuolated (VC) (G and H); toxic granulated (TG) (I and J); hypersegmented and toxic granulated (HS + TG) (K and L) neutrophils were counted. The t test was used for parametric distributed-samples (A and C), and the Mann–Whitney test was used for non-parametric analysis. The graphs are plotted as the mean ± SD. ***p < 0.0001, ** p = 0.0188, ns not statistically significant
Taken together, the results show that microcephalic children have morphological alterations in lymphocytes and neutrophils that are compatible with chronic inflammation and may impact the functionality of these cells and the whole immune response.
Microcephalic children due CZS have increased levels of IFN-γ, IL-2, IL-4, IL-5 and type I IFNs, and impaired cellular immune memory response
We further evaluated the serum concentrations of several soluble mediators of the immune system. The cytokines IFN-γ, IL-2, IL-4, IL-5, IL-6 and TNFα were measured in serum samples from 15 children with microcephaly and 16 control children by the Cytometric Bead Array (CBA) method. We observed that microcephalic children presented higher seric levels of IFN-γ (p = 0.0174; Fig. 6A), IL-2 (p = 0.0170; Fig. 6B), IL-4 (p = 0.0176; Fig. 6C) and IL-5 (p = 0.0222; Fig. 6D) even in the absence of symptomatic or asymptomatic parasitic and genitourinary infections. The cytokines IL-10 (Fig. 6E), IL-6 (Fig. 6F), TNFα (Fig. 6G) did not show significant differences between the groups. By RT-qPCR from the whole blood sample we also observed elevated levels of IFN-α and IFN-β transcripts in the microcephalic group, compared to the controls (Fig. 6H, I).
Children with microcephaly due CZS show a higher level of IFN-γ, IL-2, IL-4, IL-5, IFN-α and IFN-β than healthy children. The cytokines IFN-γ (A), IL-2 (B), IL-4 (C), IL-5 (D), IL-10 (E), IL-6 (F) and TNFα (G) were quantified in the serum of 15 children with microcephaly and 16 control children by Cytometric Bead Array. mRNA for IFN-α (H) and IFN-β (I) was assessed on 20 children with microcephaly and 16 control children by RT-qPCR. The graphs were plotted as mean ± SD. The Mann–Whitney test was used in all analyses. *p = 0.0174 (IFN-γ) *p = 0.0170 (IL-2); *p = 0.0176 (IL-4); *p = 0.0222 (IL-5); **p = 0.0061 (IFNα); *p = 0.0243 (IFNβ); ns not statistically significant
When analysing the acute inflammatory marker CRP, we observed that 45% (9 from 20 samples) of microcephalic children had a positive result and 25% (3 from 12 samples) from the control group were positive. Rheumatoid factor test was performed and no children in either group tested positive (data not pictured).
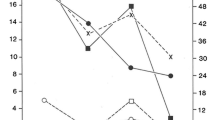
To assess the cellular immune memory in microcephalic children in vivo, we performed the tuberculin skin test on seven children previously vaccinated with BCG. It was observed that 57.12% (n = 4) of the children were not reactive to the test and 42.88% (n = 3) were weakly reactive to the PPD (Fig. 7). None of the children showed strong reactivity, as would be expected for immunocompetent children who were vaccinated up to 2 years before the tuberculin skin test, and whose expected induration diameter would be ≥ 10 mm. Together, the data showed an exacerbated and concomitant expression of Th1 and Th2 cytokines in microcephalic patients even though they do not generate an adequate T cell memory response, which can impact on increasing susceptibility to infections and changes in vaccine schedules for this population.
Children with microcephaly have low reactivity to the tuberculin skin test. Seven microcephalic children due CZS with a history of BCG vaccination had a T cell memory response assessed by the tuberculin skin test. The light grey band (bottom) represents the diameter of the non-reactive hardening (0–4 cm), the grey band (middle) corresponds to the diameter of the hardening of a weak reactor (4–10 cm) and the dark grey band (top) represents the strongly reactive hardening diameter (> 10 cm). The graph was plotted as the diameter of the hardening in cm
Discussion
Monitoring the CZS patients during their first years of life, it is possible to identify a range of symptoms in addition to the well-established neurological damage. We showed here that microcephalic children have important alterations in the size, topography and volume of lymphoid organs, increase in eosinophils numbers, morphological alterations in lymphocytes and neutrophils, increased production of IL-2, IFN-γ, IL-4, IL-5 and type I IFN in addition to an impairment in the generation of cellular immune memory for the BCG vaccine (Fig. 8). In utero ZIKV infection, besides having a great tropism to several organs [35], also triggers inflammatory reactions [36,37,38] in a site that should be hyporesponsive to allow the foetal development. It is possible that this combination, occurring during the critical period for lymphoid organogenesis, compromises the full and healthy development of the CZS patient's immune system, as we demonstrated here, which can implicate in a high susceptibility to infections after birth and low reaction to vaccines.
Congenital Zika virus syndrome leads to lymphoid organs alterations, haematological and immune dysregulation. Microcephalic children due CZS show alterations in the thymus, spleen and cervical lymph nodes; increased numbers of atypical lymphocytes, hypersegmented neutrophils and eosinophils; higher serum level of IFNα, IFNβ, IFN-γ, IL-2, IL-4 and IL-5 and impaired T lymphocyte memory response
Our study showed that 31.8% of the microcephalic children had already been diagnosed with pneumonia at some point in life, 22.7% of children were hospitalized in 2018 and 27.2% in 2019, regardless of the cause. Another study corroborates with our data, in which the caregivers of 19 children with microcephaly due CZS, aged between 19 and 24 months, reported that 42.4% of the children were previously hospitalized and 31.8% had already been diagnosed with pneumonia [39]. Another work evaluated 24 children with microcephaly due CZS during their second year of life and identified that 33.3% of the children had hospital admissions and that 12.6% of the children had been hospitalized for diarrhoea, urinary tract infection and pneumonia [40]. These high rates of hospitalizations and high numbers of pneumonia diagnoses might be related to immature or dysregulated immune system functions.
Considering that the lymphoid organs start their development during the intrauterine period and that it is possible that congenital infections might interfere in their organogenesis, we evaluated the morphometric characteristics of the thymus, spleen and cervical lymph nodes in children with microcephaly. Under normal and healthy conditions, the thymus descends during foetal life from the upper neck to its retrosternal location [13]; however, 81% of CZS children examined here presented the thymus above the level of the wishbone, possibly due to hyperplasia. One child presented coarse and diffusely heterogeneous hypoechoic echotexture, suggesting fibrosis. The thymus is responsible for the maturation of lymphoid precursors into T cells, and it is fundamental to establish the T cell pool. Thymic hyperplasia can be associated with several diseases, usually autoimmune disorders, more frequently myasthenia gravis and others less frequently, such as Graves’ disease, Addison’s disease, systemic lupus erythematosus, scleroderma and rheumatoid arthritis [41]. Without the appropriate inductive thymic environment, T cells cannot mature correctly, and both cell-mediated immunity and the production of T cell-dependent antibodies are impaired. This would lead to a compromised immune response, which must be evaluated in CZS child patients in future studies.
We also identified that more than half of the children with microcephaly had an enlarged spleen, which is an indication of splenomegaly. Usually, enlargement of the spleen is caused by infection, autoimmune disease, portal hypertension, cardiac insufficiency, haemolytic anaemia and metabolic disease [16, 42]. Besides the spleen, we also observed an enlargement of the lymph node diameter in those patients, which is usually related to inflammatory diseases [15] or with the course of infections. All the patients recruited for our study, however, had no signs of infection and tested negative for asymptomatic urinary and digestive tract infections. In addition, a study showed that children with CZS have an imbalanced immune activation and inflammation, with an increment in the systemic levels of proinflammatory cytokines [11]. Thus, the larger size observed in cervical lymph nodes could be a consequence of chronic high levels of pro-inflammatory cytokine production. The alterations in the primary and secondary lymphoid organs can be associated with changes in other immune compartments and may affect the whole immune network.
The quantitative and qualitative diagnosis based on the cellular components of the blood is important because blood cells are easily accessible indicators of disturbances in their organs of origin or degradation. Performing the complete blood count in the CZS children, we noted that eosinophilia was presented in 8 out of 13 children, even in the absence of parasitic infection. Eosinophilia may arise during allergic conditions such as asthma, urticaria, atopic dermatitis, eosinophilic esophagitis, drug reactions, dermatologic conditions, malignancies, connective tissue disease, idiopathic hypereosinophilic syndrome, or parasitic infections, including helminths [43].
When performing the differential leukocyte count, we were able to see that the CZS children presented an increase in atypical lymphocytes and also in hypersegmented neutrophils. Some studies show that atypical lymphocytes can be found in peripheral blood in response to some antigenic stimuli [44, 45]. At inflammation sites, they act like normal lymphocytes. However, morphologically, these cells are enlarged and the nucleus may have an unconventional location, size or shape. The most recent studies suggest that these atypical lymphocytes are activated T lymphocytes produced in response to infected B lymphocytes [44]. Elevated absolute lymphocyte counts, often along with cell morphology alterations, are observed predominantly in viral infections, hypersensitivity reactions or lymphocytic malignancies [44]. Furthermore, the presence of hypersegmented neutrophils can indicate dysregulated bone marrow activation, typical during megaloblastic anemias, although sometimes occurring during iron deficiency anemia [46]. Meanwhile, toxic granulation can be used as an early indicator of acute bacterial infections [47], being also a useful marker for Kawasaki disease, an acute febrile systemic vasculitis [48, 49].
To investigate whether the immunological alterations in lymphoid organs and leukocytes could result in changes in the functionality of the immune system, influencing the inflammatory mediator homeostasis, we quantified the serum concentrations of the cytokines IFNα, IFNβ, IFN-γ, IL-2, IL-4, IL-5, IL-6 and TNF-α. Curiously, we detected increased concentrations of IFNα, IFNβ, IFN-γ, IL-2, IL-4 and IL-5 in CZS children compared with healthy children. Studies that evaluated the in situ immune response profile of fatal CZS cases observed a predominance of cytokines with a Th2 profile in brain tissue [50]. Another group reported the overexpression of IL-1β, IL-18 and IL-33 in brain tissue from microcephalic children [51]. A study comparing CZS newborns, newborns with asymptomatic infection by ZIKV and uninfected newborns observed an inflammatory signature associated with CZS that suggested an increase in inflammation characterized by overexpression of various markers such as IFN-γ, IFN-α2, CXCL10, IL-1α, TNF-α, CCL3, CCL4, IL-15, IL-10, IL-6, IL-1RA and IL-8, when compared to uninfected newborns [11]. In the latter, while they did not see an increase in IL-2, IL-4 and IL-5, the samples were collected from umbilical cord at birth, suggesting that the increase we identified here might arise later, after the neonatal period.
A sustained CNS inflammation was also found in ZIKV-confirmed microcephaly cases after birth, when IFN-α, CXCL10 and CXCL9 were still elevated in the cerebrospinal fluid [52]. The authors propose that type-I IFNs have an important role in ZIKV-associated developmental defects, as a result of chronic inflammation.
In the population that we analysed, we observed that 45 and 25% of CZS children and control group, respectively, were positive for CRP, a protein synthesized by the liver, which increases in response to inflammation [53]. On the other hand, all the subjects were negative to RF, frequently found elevated in patients with rheumatic diseases and some non-rheumatic diseases, especially chronic infections such as hepatitis C, tuberculosis and subacute infective endocarditis.
Finally, to assess the functionality of memory cells in CZS children, a tuberculin skin test was performed. This test triggers a late-type hypersensitivity reaction, promoting the activation of memory T lymphocytes in patients previously exposed to the antigen that is being administered [54]. In the case of the study, all children, aged up to 2 years, with CZS were vaccinated for BCG up to 2 years, and PPD was then applied to 7 microcephalic children. We observed that 75% of patients were not reactive to PPD and 25% had low reactivity, showing that CZS children were not able to properly activate the memory T cells induced by the BCG vaccine. This result is the opposite of what is expected for BCG-vaccinated individuals, especially in those recently immunized, in which medium-sized reactions that can reach 10 mm or more are expected [54].
Consistent with the data we show here, it is reported in the literature that different types of congenital infections in humans or experimental models can cause immunological changes in the neonate and child. After Influenza virus or Mycoplasma arthritidis infection in pregnant mice, the newborn’s lymphoproliferative response to mitogenic/superantigenic introduced during pregnancy was suppressed or absent, but their immune system recognized syngeneic stimuli [55]. Human congenital syphilis was described to cause immune complex nephritis and high levels of circulating helper T cells in the child [56]. Studies carried out during the foetal phase with samples collected by cordocentesis in IGM + pregnant woman for rubella virus, cytomegalovirus or parvovirus showed trombocitopenie, elevated lymphocyte count and increase in NK cells in the foetal phase, but the extension of the immunological and hematological changes to the postnatal period was not evaluated in this study [57]. Another work shows that suppression of immunological response in a newborn with congenital CMV infection includes thrombocytopenia, leukopenia, hepatosplenomegaly and chronic pneumonia [58]. Children vertically infected by HIV have immunological changes including hypergammaglobulinemia; diminished counts of T CD4 + , CD16 + /CD56 + and CD19 + cells, memory CD4 + T cells and CD4 + /CD8 + ratio; impaired proliferative response to mitogens and antigens and lower production of IL-2 and IFN compared to the controls [59]. Furthermore, perinatal chlamydial infection results in newborns with decreased numbers of T and B lymphocytes, decrease in CD4/CD8 ratio and increased level of IgM in comparison with the healthy newborns [60].
Our data bring to light new symptoms in CZS children. As a consequence of the ZIKV congenital infection, these patients present thymic, splenic and cervical lymph nodes hyperplasia, an increase in eosinophils numbers, lymphocytes and neutrophils morphological alterations, increased production of IL-2, IFN-γ, IL-4, IL-5 and type I IFN and impaired T lymphocyte memory response. Altogether, these findings demonstrate an immunological imbalance in microcephalic children with congenital Zika virus syndrome and suggest some level of impairment in the immune function of CZS children. These alterations should be investigated in children with other congenital infections. Whether these children have a greater susceptibility to infections and a possible Th2-skewed immune response should be further investigated, as well as a better adequate vaccination scheme.
References
Dick GWA, Kitchen SF, Haddow AJ (1952) Zika virus. I. isolations and serological specificity. Trans R Soc Trop Med Hyg 46:509–520. https://doi.org/10.1016/0035-9203(52)90042-4
Zanluca C, de Melo VCA, Mosimann ALP et al (2015) First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 110:569–572. https://doi.org/10.1590/0074-02760150192
Campos GS, Bandeira AC, Sardi SI (2015) Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis 21:1885–1886. https://doi.org/10.3201/eid2110.150847
Calvet G, Aguiar RS, Melo ASO et al (2016) Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 16:653–660. https://doi.org/10.1016/S1473-3099(16)00095-5
Mlakar J, Korva M, Tul N et al (2016) Zika Virus Associated with Microcephaly. N Engl J Med 374:951–958. https://doi.org/10.1056/NEJMoa1600651
Eickmann SH, Carvalho MDCG, Ramos RCF et al (2016) Zika virus congenital syndrome. Cad Saude Publica. https://doi.org/10.1590/0102-311X00047716
van der Linden V, Pessoa A, Dobyns W et al (2016) Description of 13 infants born during october 2015-January 2016 with congenital Zika virus infection without microcephaly at birth-Brazil. MMWR Morb Mortal Wkly Rep 65:1343–1348. https://doi.org/10.15585/mmwr.mm6547e2
Pan American Health Organization/World Health Organization (2016) PAHO/WHO updates the characterization of Zika congenital syndrome. In: Pan Am. Health Organ. World Health Organ. https://www3.paho.org/hq/index.php?option=com_content&view=article&id=12318:pahowho-updates-characterization-zika-congenital-syndrome&Itemid=1926&lang=en. Accessed 26 Nov 2021
Faizan MI, Abdullah M, Ali S et al (2016) Zika virus-induced microcephaly and its possible molecular mechanism. Intervirology 59:152–158. https://doi.org/10.1159/000452950
Cavalcanti DD, Alves LV, Furtado GJ et al (2017) Echocardiographic findings in infants with presumed congenital Zika syndrome: Retrospective case series study. PLoS One 12:e0175065. https://doi.org/10.1371/journal.pone.0175065
Vinhaes CL, Arriaga MB, de Almeida BL et al (2020) Newborns with Zika virus-associated microcephaly exhibit marked systemic inflammatory imbalance. J Infect Dis. https://doi.org/10.1093/infdis/jiaa197
Holt PG, Jones CA (2000) The development of the immune system during pregnancy and early life. Allergy 55:688–697. https://doi.org/10.1034/j.1398-9995.2000.00118.x
Sauce D, Appay V (2011) Altered thymic activity in early life: how does it affect the immune system in young adults? Curr Opin Immunol 23:543–548. https://doi.org/10.1016/j.coi.2011.05.001
Pearse G (2006) Normal structure, function and histology of the thymus. Toxicol Pathol 34:504–514. https://doi.org/10.1080/01926230600865549
Willard-Mack CL (2006) Normal structure, function, and histology of lymph nodes. Toxicol Pathol 34:409–424. https://doi.org/10.1080/01926230600867727
Cesta MF (2006) Normal structure, function, and histology of the spleen. Toxicol Pathol 34:455–465. https://doi.org/10.1080/01926230600867743
Davies NP, Buggins AG, Snijders RJ et al (1992) Blood leucocyte count in the human fetus. Arch Dis Child 67:399–403
Comans-Bitter WM, de Groot R, van den Beemd R et al (1997) Immunophenotyping of blood lymphocytes in childhood reference values for lymphocyte subpopulations. J Pediatr 130:388–393. https://doi.org/10.1016/s0022-3476(97)70200-2
Walker JC, Smolders MAJC, Gemen EFA et al (2011) Development of lymphocyte subpopulations in preterm infants. Scand J Immunol 73:53–58. https://doi.org/10.1111/j.1365-3083.2010.02473.x
Sautois B, Fillet G, Beguin Y (1997) Comparative cytokine production by in vitro stimulated mononucleated cells from cord blood and adult blood. Exp Hematol 25:103–108
Cupedo T (2011) Human lymph node development: an inflammatory interaction. Immunol Lett 138:4–6. https://doi.org/10.1016/j.imlet.2011.02.008
Saghafian-Hedengren S, Sundström Y, Sohlberg E et al (2009) Herpesvirus seropositivity in childhood associates with decreased monocyte-induced NK Cell IFN-γ production. J Immunol 182:2511–2517. https://doi.org/10.4049/jimmunol.0801699
Machado A (2014) Análise do perfil imunológico em recém nascidos com toxoplasmose congênita apresentando diferentes formas clínicas da doença ocular. Universidade Federal de Minas Gerais
Secretaria de Vigilância em Saúde (2017) Vírus Zika no Brasil: a resposta do SUS, 1st edn. Ministério da Saúde, Brasília
Araújo TVB, de Ximenes RA, de Barros M-FD et al (2018) Association between microcephaly, Zika virus infection, and other risk factors in Brazil: final report of a case-control study. Lancet Infect Dis 18:328–336. https://doi.org/10.1016/S1473-3099(17)30727-2
Silva AA, Barbieri MA, Alves MT et al (2018) Prevalence and risk factors for microcephaly at birth in Brazil in 2010. Pediatrics 141:e20170589. https://doi.org/10.1542/peds.2017-0589
Moore CA, Staples JE, Dobyns WB et al (2017) Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr 171:288–295. https://doi.org/10.1001/jamapediatrics.2016.3982
Faye O, Faye O, Diallo D et al (2013) Quantitative real-time PCR detection of Zika virus and evaluation with field-caught Mosquitoes. Virol J 10:311. https://doi.org/10.1186/1743-422X-10-311
Pelizzo G, Guazzotti M, Klersy C et al (2018) Spleen size evaluation in children: time to define splenomegaly for pediatric surgeons and pediatricians. PLoS One 13:e0202741. https://doi.org/10.1371/journal.pone.0202741
Riccabona M (2014) Pediatric ultrasound. Springer, Berlin, Heidelberg
Beek E, van Rijn RR, Daneman A (2016) Diagnostic pediatric ultrasound, 1st edn. Thieme, Stuttgart
Robben SG, Lequin MH, Diepstraten AF et al (1999) Anterior joint capsule of the normal hip and in children with transient synovitis: US study with anatomic and histologic correlation. Radiology 210:499–507. https://doi.org/10.1148/radiology.210.2.r99fe52499
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods San Diego Calif 25:402–408. https://doi.org/10.1006/meth.2001.1262
Júnior A, Filho M, Reis R (2010) Urologia fundamental. Planmark, São Paulo
El Costa H, Gouilly J, Mansuy J-M et al (2016) ZIKA virus reveals broad tissue and cell tropism during the first trimester of pregnancy. Sci Rep 6:35296. https://doi.org/10.1038/srep35296
Panganiban AT, Blair RV, Hattler JB et al (2020) A Zika virus primary isolate induces neuroinflammation, compromises the blood-brain barrier and upregulates CXCL12 in adult macaques. Brain Pathol 30:1017–1027. https://doi.org/10.1111/bpa.12873
White MK, Wollebo HS, Beckham JD et al (2016) Zika virus: an emergent neuropathological agent. Ann Neurol 80:479–489. https://doi.org/10.1002/ana.24748
Enlow W, Bordeleau M, Piret J et al (2021) Microglia are involved in phagocytosis and extracellular digestion during Zika virus encephalitis in young adult immunodeficient mice. J Neuroinflammation 18:178. https://doi.org/10.1186/s12974-021-02221-z
Satterfield-Nash A, Kotzky K, Allen J et al (2017) Health and development at age 19–24 months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika virus infection during the 2015 Zika virus outbreak-Brazil, 2017. MMWR Morb Mortal Wkly Rep 66:1347–1351. https://doi.org/10.15585/mmwr.mm6649a2
Alves LV, Paredes CE, Silva GC et al (2018) Neurodevelopment of 24 children born in Brazil with congenital Zika syndrome in 2015: a case series study. BMJ Open 8:e021304. https://doi.org/10.1136/bmjopen-2017-021304
Kumar V, Abbas AK, Aster JC, Perkins JA (2018) Robbins basic pathology, 10th edn. Elsevier, Philadelphia, Pennsylvania
McKenzie CV, Colonne CK, Yeo JH, Fraser ST (2018) Splenomegaly: Pathophysiological bases and therapeutic options. Int J Biochem Cell Biol 94:40–43. https://doi.org/10.1016/j.biocel.2017.11.011
Kovalszki A, Weller PF (2016) Eosinophilia. Prim Care 43:607–617. https://doi.org/10.1016/j.pop.2016.07.010
Riley LK, Rupert J (2015) Evaluation of patients with leukocytosis. Am Fam Physician 92:1004–1011
Lv J, Gao M, Zong H et al (2020) Application of peripheral blood lymphocyte count in prediction of the presence of atypical lymphocytes. Clin Lab. https://doi.org/10.7754/Clin.Lab.2019.191113
Sipahi T, Tavil B, Unver Y (2002) Neutrophil hypersegmentation in children with iron deficiency anemia. Pediatr Hematol Oncol 19:235–238. https://doi.org/10.1080/08880010252899398
Al-Gwaiz LA, Babay HH (2007) The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med Princ Pract 16:344–347. https://doi.org/10.1159/000104806
Birdi N, Klassen TP, Quinlan A et al (1999) Role of the toxic neutrophil count in the early diagnosis of Kawasaki disease. J Rheumatol 26:904–908
Agarwal S, Agrawal DK (2017) Kawasaki disease: etiopathogenesis and novel treatment strategies. Expert Rev Clin Immunol 13:247–258. https://doi.org/10.1080/1744666X.2017.1232165
Azevedo RSS, de Sousa JR, Araujo MTF et al (2018) In situ immune response and mechanisms of cell damage in central nervous system of fatal cases microcephaly by Zika virus. Sci Rep 8:1. https://doi.org/10.1038/s41598-017-17765-5
de Sousa JR, Azevedo RSS, Martins Filho AJ et al (2018) Correlation between apoptosis and in situ immune response in fatal cases of microcephaly caused by Zika virus. Am J Pathol 188:2644–2652. https://doi.org/10.1016/j.ajpath.2018.07.009
Lima MC, de Mendonça LR, Rezende AM et al (2019) The Transcriptional and protein profile from human infected neuroprogenitor cells is strongly correlated to Zika virus microcephaly cytokines phenotype evidencing a persistent inflammation in the CNS. Front Immunol 10:1–14
Nehring SM, Goyal A, Patel BC (2022) C reactive protein StatPearls. StatPearls Publishing, Treasure Island (FL)
Ministério da saúde (2014) Técnicas de aplicação e leitura da prova tuberculínica, 1st ed. Brasília
Pronin AV, Deeva AV, Zuev VA et al (1997) The immunological consequences of congenital infections. Zh Mikrobiol Epidemiol Immunobiol 105–108
Chen WP, Chiang H, Lin CY (1988) Persistent histological and immunological abnormalities in congenital syphilitic glomerulonephritis after disappearance of proteinuria. Child Nephrol Urol 9:93–97
Michie CA, Harvey D (1995) Fetal immunological and haematological changes in intrauterine infection. BJOG Int J Obstet Gynaecol 102:79–79. https://doi.org/10.1111/j.1471-0528.1995.tb09041.x
Furmaga-Jabłońska W, Korczowski B, Maksymiuk B (1996) Suppression of immunological response in a newborn with congenital cytomegalovirus infection: diagnostic difficulties. Pediatr Pol 71:351–357
García Rodríguez MC (1998) Immunological changes in the child infected by vertical HIV transmission. Allergol Immunopathol (Madr) 26:129–135
Pirogova VI, Vinograd NA, Zhemela EM, Chegrinec NA (1995) Immune status of newborns in perinatal chlamydial infection. Am J Reprod Immunol 33:94–96. https://doi.org/10.1111/j.1600-0897.1995.tb01144.x
Acknowledgements
This work was supported by the National Council for Scientific and Technological Development–CNPq [Grant no 431941/2018-1 and no 311055/2019-2]; the Brazilian Federal Agency for Support and Evaluation of Graduate Education–CAPES; and the Santos Dumont Institute-Ministry of Education of Brazil. The group thanks Fernanda Mesquita, Kim Yano, Erianna Macedo, Luciana de Morais, Rízia Silva, Maria Evanísia Calheiros and Rute Mendonça for technical assistance. The most special acknowledgments are to the families, mothers, fathers and children who agreed to participate in this research and who often adapted their routines so the research could be performed.
Author information
Authors and Affiliations
Contributions
ACAS: Conceptualization, Methodology, Formal analysis, Investigation, Writing—Original Draft, Writing—Review & Editing and Visualization. WPB: Conceptualization, Methodology, Formal analysis, Writing—Original Draft, Writing—Review & Editing and Visualization. RLLdeS: Investigation—Review & Editing. LCP: Investigation—Review & Editing. LMdoN: Investigation—Review & Editing. ACCCB: Investigation, Methodology—Review & Editing. MNS: Investigation, Resources—Review & Editing. LECS: Investigation—Review & Editing. VaAdeA: Methodology—Review & Editing. HdeSP: Methodology—Review & Editing. VSdeFS: Investigation, Formal analysis—Review & Editing. CMB: Investigation—Review & Editing. RAdeOFJ: Conceptualization, Investigation, Writing—Review & Editing, Funding acquisition. PMdaMG: Conceptualization, Investigation, Writing—Review & Editing. TdeSLK: Conceptualization, Investigation, Writing—Review & Editing. MSLN: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing—Original Draft, Writing—Review & Editing and Visualization, Supervision, Project administration and Funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Edited by Christian Bogdan.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salmeron, A.C.A., Bezerra, W.P., de Souza, R.L.L. et al. Immunological imbalance in microcephalic children with congenital Zika virus syndrome. Med Microbiol Immunol 211, 219–235 (2022). https://doi.org/10.1007/s00430-022-00746-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-022-00746-5