Abstract
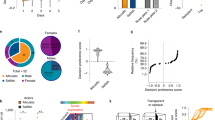
Recent evidence suggests that increased dopaminergic signaling within the dorsal striatum played a central role in the evolution of the human brain. This increase has been linked to human prosociality and language in what has been described as a dopamine-dominated striatum personality style. Increased striatal dopamine is associated with an increase in ventral striatal activity and promotes externally driven behaviors, including cooperation and social conformity. In contrast, decreased striatal dopamine is associated with increased dorsal striatal activity and favors internally driven and goal-oriented behaviors. Previous comparative studies have focused on the dorsal striatum, measuring dopaminergic innervation in the dorsal and medial caudate nucleus and putamen. Here, we add to this knowledge by examining regions of the ventral striatum. We quantified the density of tyrosine hydroxylase–immunoreactive axons, as a measure of dopaminergic innervation, in the nucleus accumbens and ventral pallidum of humans, great apes, platyrrhine and cercopithecid monkeys. Our data show that humans have a significantly greater dopaminergic innervation in both structures, supporting the hypothesis that selection for a prosocial neurochemistry in the human basal ganglia may have contributed to the evolution of our uniquely social behavior profile.









Similar content being viewed by others
Availability of data and materials
All raw data are available and will be posted in the National Chimpanzee Brain Resource website data repository (https://www.chimpanzeebrain.org/data-repository) and can be requested from the corresponding authors.
Code availability
NA.
References
Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z (2006) Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neurosci 9:133–139
Arias-Carrión O, Stamelou M, Murillo-Rodriguez E, Menéndez-González M, Pöppel E (2010) Dopaminergic reward system: a short integrative review. Int Arch Med 3:24
Báez-Mendoza R, Schultz W (2013) The role of the striatum in social behavior. Front Neurosci 7:233
Báez-Mendoza R, Harris CJ, Schultz W (2013) Activity of striatal neurons reflects social action and own reward. Proc Nat Acad Sci USA 110:16634–16639
Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP (2007) Neural correltaes of pair-bonding in a monogamous primate. Brain Res 1184:245–253
Bergey CM, Phillips-Conroy JE, Disotell TR, Jolly CJ (2016) Dopamine pathway is highly diverged in primate species that differ markedly in social behavior. Proc Natl Acad Sci USA 113:6178–6181
Calvey T (2019) Human self-domestication and the extended evolutionary synthesis of addiction: how humans evolved a unique vulnerability. Neuroscience 419:100–107
Cools AR, Hendriks G, Korten J (1975) The acetylcholine-dopamine balance in the basal ganglia of rhesus monkeys and its role in dynamic, dystonic, dyskinetic, and epileptoid motor activities. J Neural Transm 36:91–105
Cools AR, Brachten R, Heeren D, Willemen A, Ellenbroek B (1990) Search after neurobiological profile of individual-specific features of Wistar rats. Brain Res Bull 24:49–69
Curtis JT, Liu Y, Aragona BJ, Wang Z (2006) Dopamine and monogamy. Brain Res 1126:76–90
Filkowski MM, Cochran RN, Haas BW (2016) Altruistic behavior: mapping responses in the brain. Neurosci Neuroecon 5:65–75
Haber SN (2003) The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 26:317–330
Haber SN (2014) The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 282:248–257
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy to human imaging. Neuropsychopharmacology 35:4–26
Haber SN, McFarland NR (1999) The concept of the ventral striatum in nonhuman primates. Ann NY Acad Sci 877:33–48
Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E (1995) The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci 15:4851–4867
Haber SN, Kim K-S, Mailly P, Cazavara R (2006) Reward-related cortical inputs define a larger striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci 26:8368–8376
Haug H (1987) Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant). Am J Anat 180:126–142
Hawkins A, Olszewski J (1957) Glia/nerve cell index for cortex of the whale. Science 126:76–77
Herculano-Houzel S (2014) The glia/neuron ratio: How it varies uniformly across brain structures and species and what it means for brain physiology and evolution. Glia 62:1377–1391
Higley JD, King ST Jr, Hasert MF, Champoux M, Suomi SJ, Linnoila M (1996) Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in rhesus macaque females. Neuropsychopharmacol 14:67–76
Holloway RL (1967) Tools and teeth: some speculations regarding canine reduction. Am Anthropol 69:63–67
Hostetler CM et al (2017) Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus). Am J Primatol 79:e22612
Inoue-Murayama M et al (2002) Variation of variable number of tandem repeat sequences in the 3′-untranslated region of primate dopamine transporter genes that affects reporter gene expression. Neurosci Lett 334:206–210
Jolly CJ, Phillips-COnroy JE, Kaplan JR, Mann JJ (2008) Cerebrospinal fluid monoaminergic metabolites in wild Papio anubis and P. hamadryas are concordant with taxon-specific behavioral ontogeny. Int J Primatol 29:1549–1566
Jolly CJ, Phillips-Conroy JE, Kaplan JR, Mann JJ (2013) Monoamine neurotransmitter metabolites in the cerebrospinal fluid of a group of hybrid baboons (Papio hamadryas and P. anubis). Int J Primatol 34:836–858
Kalivas PW, Volkow ND (2005) The neural basis of addition: a pathology of motivation and choice. Am J Psychiatry 162:1403–1413
Klimecki OM, Leiberg S, Ricard M, Singer T (2014) Differential pattern of functional brain plasticity after compassion and empathy training. Soc Cogn Affect Neurosci 9:873–879
Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G (2009) Reinforcement learning signal predicts social conformity. Neuron 61:140–151
Lovejoy CO (1981) The origin of man. Science 211:341–350
Lovejoy CO (2009) Reexamining human origins in light of Ardipithecus ramidus. Science 326:74e71-74e78
Lovejoy CO (2014) Ardipithecus and early human evolution in light of twenty-first-century developmental biology. J Anthropol Res 70:337–363
Mavridis I (2015) The role of the nucleus accumbens in psychiaric disorders. Psychiatriki 25:282–294
McCollum LA, McCullumsmith RE, Roberts RC (2016) Tyrosine hydroxylase localization in the nucleus accumbens in schizophrenia. Brain Sruct Funct 221:4451–4458
Miller GM, Madras BK (2002) Polymorphisms in the 3’-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry 7:44–55
Nowak RM (1999) Walker’s primates of the world. The Johns Hopkins University Press, Baltimore
O’Connell LA, Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol 519:3599–3639
Paxinos G et al (2000) The rhesus monkey brain in stereotaxic coordinates. Academic Press, San Diego, CA
Paxinos G, Watson C, Petrides M, Rosa M, Tokuno H (2012) The marmoset brain in stereotaxic coordinates. Academic Press/Elsevier
Phillips KA et al (2014) Why primate models matter. Am J Primatol 76:801–827
Preuss TM (2019) Critique of pure marmoset. Brain Behav Evol 93:92–107
Raghanti MA, Stimpson CD, Marcinkiewicz JL, Erwin JM, Hof PR, Sherwood CC (2008) Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Neuroscience 155:203–220
Raghanti MA et al (2009) Species-specific distributions of tyrosine hydroxylase-immunoreactive neurons within the prefrontal cortex of anthropoid primates. Neuroscience 158:1551–1559
Raghanti MA et al (2016) Human-specific increase in dopaminergic innervation in a striatal region associated with speech and language: a comparative analysis of the primate basal ganglia. J Comp Neurol 524:2117–2129
Raghanti MA et al (2018) A neurochemical hypothesis for the origin of hominids. Proc Natl Acad Sci 1719666115:E1719661108–E1719661116. https://doi.org/10.1073/pnas.1719666115
Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A (1991) Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Res 559:181–190
Rilling JK, Sanfey AG (2011) The neuroscience of social decision making. Annu Rev Psychol 62:23–48
Sherwood CC et al (2006) Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA 103:13606–13611
Smaers JB, Rohlf FJ (2016) Testing species’ deviation from allometric predictions using the phylogenetic regression. Evolution 70:1145–1149
Sousa AMM et al (2017) Molecular and cellular reorganization of neural circuits in the human lineage. Science 358:1027–1032
Stallen M, Sanfey AG (2015) The neuroscience of social conformity: implications for fundamental and applied research. Front Neurosci 9:337
Tachibana Y, Hikosaka O (2012) The primate central pallidum encodes expected reward value and regulates motor action. Neuron 76:826–837
Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S (2004) Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature Neurosci 7:887–893
Tanaka SC, Schweighofer N, Asahi S, Shishida K, Okamoto Y, Yamawaki S, Doya K (2007) Serotonin differentially regulates short- and long-term prediction of rewards in the ventral and dorsal striatum. PLoS ONE. https://doi.org/10.1371/journal.pone.0001333
van den Bos R (2015) The dorsal and ventral striatum play different roles in the programming of social behaviour: a tribute to Lex Cools. Behav Pharmacol 26:6–17
van den Bercken J, Cools AR (1982) Evidence for the role of the caudate nucleus in the sequential organization of behavior. Behav Brain Res 4:319–337
Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162:712–725
Young LJ, Wang Z (2004) The neurobiology of pair bonding. Nat Neurosci 7:1048–1054
Young LJ, Wang Z, Insel TR (1998) Neuroendocrine bases of monogamy. Trends Neurosci 2:71–75
Zaki J, Schirmer J, Mitchell JP (2011) Social influence modulates the neural computation of value. Psychol Sci 22:894–900
Acknowledgements
This research was funded by the National Science Foundation (NSF BCS-1846201 to MAR and SMA-1542848 and EF-2021785 to CCS and WDH). Elaine Miller was supported by the National Science Foundation Graduate Research Fellowship Program (1746914). We would like to thank Dr. Richard S. Meindl for statistical advice. We are grateful to each of the following for the use of brain materials: The NIH NeuroBioBank, National Chimpanzee Brain Resource (NIH grant NS092988), NINDS Grants NS-402867 and NS0-73134, The Great Ape Aging Project (supported by NIH Grant AG014308), the National Primate Research Center at the University of Washington (NIH grant RR000166), the Oregon National Primate Research Center (NIH P51 OD011092), the Northwestern University Alzheimer’s Disease Center Brain Bank (supported by Alzheimer’s Disease Core Center Grant AG013854, from the National Institute on Aging to Northwestern University, Chicago, IL).
Funding
This research was funded by the National Science Foundation (NSF BCS-1846201 to MAR and SMA-1542848 and EF-2021785 to CCS and WDH). Elaine Miller was supported by the National Science Foundation Graduate Research Fellowship Program (1746914).
Author information
Authors and Affiliations
Contributions
KH and MAR contributed to the study conception and design. Material preparation, data collection and analysis were performed by KH, EM, CDS, KAP, WDH, PRH, CCS, COL, and MAR. The first draft of the manuscript was written by KH and MAR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
None.
Ethics approval
Human postmortem brain samples meet the criteria for IRB Exemption 4 under DHHS regulations 45 CFR 46 (46.101). Because no living subjects are involved in this research, this proposal does not qualify for IACUC oversight. However, when alive, all nonhuman primates were housed at research or zoological institutions and were maintained in accordance with each institution’s animal care and use guidelines.
Consent to participate
NA.
Consent for publication
NA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hirter, K.N., Miller, E.N., Stimpson, C.D. et al. The nucleus accumbens and ventral pallidum exhibit greater dopaminergic innervation in humans compared to other primates. Brain Struct Funct 226, 1909–1923 (2021). https://doi.org/10.1007/s00429-021-02300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02300-0