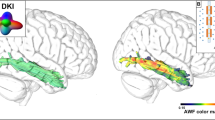
Abstract
In speech production, an important step before motor programming is the retrieval and encoding of the phonological elements of target words. It has been proposed that phonological encoding is supported by multiple regions in the left frontal, temporal and parietal regions and their underlying white matter, especially the left arcuate fasciculus (AF) or superior longitudinal fasciculus (SLF). It is unclear, however, whether the effects of AF/SLF are indeed related to phonological encoding for output and whether there are other white matter tracts that also contribute to this process. We comprehensively investigated the anatomical connectivity supporting phonological encoding in production by studying the relationship between the integrity of all major white matter tracts across the entire brain and phonological encoding deficits in a group of 69 patients with brain damage. The integrity of each white matter tract was measured both by the percentage of damaged voxels (structural imaging) and the mean fractional anisotropy value (diffusion tensor imaging). The phonological encoding deficits were assessed by various measures in two oral production tasks that involve phonological encoding: the percentage of nonword (phonological) errors in oral picture naming and the accuracy of word reading aloud with word comprehension ability regressed out. We found that the integrity of the left SLF in both the structural and diffusion tensor imaging measures consistently predicted the severity of phonological encoding impairment in the two phonological production tasks. Such effects of the left SLF on phonological production remained significant when a range of potential confounding factors were considered through partial correlation, including total lesion volume, demographic factors, lesions on phonological-relevant grey matter regions, or effects originating from the phonological perception or semantic processes. Our results therefore conclusively demonstrate the central role of the left SLF in phonological encoding in speech production.



Similar content being viewed by others
Notes
Note that although AF and SLF have been traditionally viewed as synonyms depicting the same tract (see Dick and Tremblay 2012 for a review), there are recent debates about their distinctions (Makris et al. 2005; Petrides and Pandya 2009; Schmahmann et al. 2007). We for now do not distinguish them to cover studies about these tracts more comprehensively and defer their potential differences to the discussion.
References
Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S et al (1999) Conduction aphasia and the arcuate fasciculus: a reexamination of the Wernicke-Geschwind model. Brain Lang 70(1):1–12
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111(3):209–219
Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003) Voxel-based lesion–symptom mapping. Nat Neurosci 6(5):448–450
Bi Y, Han Z, Weekes B, Shu H (2007) The interaction between semantic and the nonsemantic systems in reading: evidence from Chinese. Neuropsychologia 45(12):2660–2673
Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC (2008) Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. Am J Neuroradiol 29(3):483–487
Brunner E, Munzel U (2000) The nonparametric Behrens-Fisher problem: asymptotic theory and a small-sample approximation. Biom J 42(1):17–25
Buchsbaum BR, Hickok G, Humphries C (2001) Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cogn Sci 25(5):663–678
Caramazza A (1997) How many levels of processing are there in lexical access? Cogn Neuropsychol 14(1):177–208
Compston A (2006) From the archives. Brain 129(6):1347–1350
Cui Z, Zhong S, Xu P, He Y, Gong G (2013) PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 7:42
Dell GS (1986) A spreading-activation theory of retrieval in sentence production. Psychol Rev 93(3):283–321
Dick AS, Tremblay P (2012) Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain 135(12):3529–3550
Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP et al (2002) Intraoperative mapping of the subcortical language pathways using direct stimulations. Brain 125(1):199–214
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Forster KI, Forster JC (2003) DMDX: a Windows display program with millisecond accuracy. Behav Res Method Instrum Comp 35(1):116–124
Fridriksson J, Kjartansson O, Morgan PS, Hjaltason H, Magnusdottir S, Bonilha L, Rorden C (2010) Impaired speech repetition and left parietal lobe damage. J Neurosci 30(33):11057–11061
Friederici AD (2009) Pathways to language: fiber tracts in the human brain. Tr Cogn Sci 13(4):175–181
Gagnon DA, Schwartz MF, Martin N, Dell GS, Saffran EM (1997) The origins of formal paraphasias in aphasics’ picture naming. Brain Lang 59(3):450–472
Gao SR (1993) Aphasia. Beijing Medicine University and China Xiehe Medicine University Joint Press, Beijing
Geschwind N (1965) Disconnexion syndromes in animals and man. Brain 88(3):585–644
Goebell E, Paustenbach S, Vaeterlein O, Ding XQ, Heese O, Fiehler J et al (2006) Low-grade and anaplastic gliomas: differences in architecture evaluated with diffusion-tensor MR imaging. Radiology 239(1):217–222
Han Z, Ma Y, Gong G, He Y, Caramazza A, Bi Y (2013) White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain 136(Pt 10):2952–2965
Hickok G, Poeppel D (2004) Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 92(1–2):67–99
Hickok G, Poeppel D (2007) The cortical organization of speech processing. Nat Rev Neurosci 8(5):393–402
Hillis AE, Caramazza A (1995) Converging evidence for the interaction of semantic and sublexical phonological information in accessing lexical representations for spoken output. Cogn Neuropsychol 12(2):187–227
Howard D, Patterson K (1992) Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Pictures. Thames Valley Test Company, Bury St Edmunds
Indefrey P (2011) The spatial and temporal signatures of word production components: a critical update. Front Psychol 2:255
Indefrey P, Levelt WJ (2004) The spatial and temporal signatures of word production components. Cognition 92(1):101–144
Lazar M, Alexander AL, Thottakara PJ, Badie B, Field AS (2006) White Matter Reorganization After Surgical Resection of Brain Tumors and Vascular Malformations. Am J Neuroradiol 27(6):1258–1271
Levelt WJ (1999) Models of word production. Tr Cogn Sci 3(6):223–232
Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Pandya DN (2005) Segmentation of Subcomponents within the Superior Longitudinal Fascicle in Humans: a Quantitative, In Vivo DT-MRI Study. Cereb Cortex 15(6):854–869
Maldonado IL, Moritz-Gasser S, Duffau H (2011) Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Fun 216(3):1–12
Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H (2007) Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130(3):623–629
Marchina S, Zhu LL, Norton A, Zipse L, Wan CY, Schlaug G (2011) Impairment of speech production predicted by lesion load of the left arcuate fasciculus. Stroke 42(8):2251–2256
Mendez MF, Benson D (1985) Atypical conduction aphasia: a disconnection syndrome. Arch Neurol 42(9):886–891
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9(1):97–113
Petrides M, Pandya DN (2009) Distinct parietal and temporal pathways to the homologues of Broca’s area in the monkey. PLoS Biol 7(8):e1000170
Rolheiser T, Stamatakis EA, Tyler LK (2011) Dynamic processing in the human language system: synergy between the arcuate fascicle and extreme capsule. J Neurosci 31(47):16949–16957
Rorden C, Karnath HO, Bonilha L (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19(7):1081–1088
Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry MS et al (2008) Ventral and dorsal pathways for language. Proc Natl Acad Sci 105(46):18035–18040
Schmahmann JD, Pandya DN, Wang R, Dai G, D’Arceuil HE, Crespigny AJ, Wedeen VJ (2007) Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130(3):630–653
Schwartz MF, Faseyitan O, Kim J, Coslett HB (2012) The dorsal stream contribution to phonological retrieval in object naming. Brain 13(Pt 12):3799–3814
Selnes OA, van Zijl PCM, Barker PB, Hillis AE, Mori S (2002) MR diffusion tensor imaging documented arcuate fasciculus lesion in a patient with normal repetition performance. Aphasiology 16(9):897–902
Shuren JE, Schefft BK, Yeh HS, Privitera MD, Cahill WT, Houston W (1995) Repetition and the arcuate fasciculus. J Neurol 242(9):596–598
Tremblay S, Shiller DM, Ostry DJ (2003) Somatosensory basis of speech production. Nature 423(6942):866–869
Vandermosten M, Boets B, Poelmans H, Sunaert S, Wouters J, Ghesquière P (2012) A tractography study in dyslexia: Neuroanatomic correlates of orthographic, phonological and speech processing. Brain 135(Pt 3):935–948
Weekes BS, Yin W, Su IF, Chen MJ (2006) The cognitive neuropsychology of reading and writing in Chinese. Lang linguist 7(3):595–617
Wernicke C (1970) The aphasic symptom complex: a psychological study on a neurological basis. Arch Neurol 22(3):280–282
Wilson SM, Galantucci S, Tartaglia MC, Rising K, Patterson DK, Henry ML et al (2011) Syntactic processing depends on dorsal language tracts. Neuron 72(2):397–403
Acknowledgments
We thank Litao Zhu for help with imaging methodology, Xiaodong Liu for help with statistical analysis and all BNU-CNLab members for data collection and imaging preprocessing, in particular Yangwen Xu, Fangson Liu and Jing Chen. We are also grateful to all research participants. This study was funded by 973 Program (2013CB837300; 2014CB846100), Major Project of National Social Science Foundation (11&ZD186), NSFC (31171073; 31222024; 31221003; 81071149; 81271548; 81371535; 31271115; 81322021), Beijing Natural Science Foundation (Z111107067311036), NCET (12-0055; 12-0065), Beijing Nova Program (Z121110002512032), Beijing New Medical Discipline Based Group (100270569). Author contributions: ZH, YB and LS designed research; ZH and YM performed research; ZH, YM and GG analyzed data; RH designed imaging sequence; LS recruited and screened patients; ZH and YB wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Z. Han, Y. Ma are co-first authors.
Rights and permissions
About this article
Cite this article
Han, Z., Ma, Y., Gong, G. et al. White matter pathway supporting phonological encoding in speech production: a multi-modal imaging study of brain damage patients. Brain Struct Funct 221, 577–589 (2016). https://doi.org/10.1007/s00429-014-0926-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0926-2