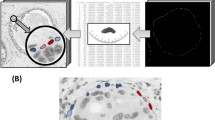
Abstract
Breast cancer is the most diagnosed cancer in humans. In recent years, myxoid and proportionated stroma have been described as clinically significant in many cancer subtypes. Here computational portraits of tumor-associated stromata were created from a machine learning (ML) classifier using QuPath to evaluate proportionated stromal area (PSA), myxoid stromal ratio (MSR), and immune stroma proportion (ISP) from whole slide images (WSI). The ML classifier was validated in independent training (n = 40) and validation (n = 109) cohorts finding MSR, PSA, and ISP to be associated with tumor stage, lymph node status, Nottingham grade, stromal differentiation (SD), tumor size, estrogen receptor (ER), progesterone receptor (PR), and receptor tyrosine-protein kinase erbB-2 (HER-2). Overall, MSR correlated better with the clinicopathologic profile than PSA and ISP. High MSR was found to be associated with high tumor stage, low ISP, and high Nottingham histologic score. As a computational biomarker, high MSR was more likely to be associated with luminal B like, Her-2 enriched, and triple-negative biomarker status when compared to luminal A like. The supervised ML superpixel approach demonstrated here can be performed by a trained pathologist to provide a faster and more uniformed approach to the analysis to the tumoral microenvironment (TME). The TME may be relevant for clinical decision-making, determining chemotherapeutic efficacy, and guiding a more overall precision-based breast cancer care.


Similar content being viewed by others
Data availability
Pathology data and the statistical analyses for the current study are available from the corresponding author upon reasonable request.
S. H. is the cofounder of CloudPath Diagnostics LLC, New York. The remaining authors declare no competing interests.
References
Lukasiewicz SCM, Forma A et al (2021) Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers MDPI 13:17
KDA Aysola, C Welch, J Xu, Y Qin, V Reddy, R Matthews, C Owens, J Okoli, D Beech, C Piyathilake, S Reddy, V Rao 2013 Triple negative breast cancer - an overview. Hereditary Genet, 2013.
Henke E, Nandigama R, Ergün S (2020) Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Frontiers in Molecular Biosciences 6:160
Kramer CVK, Pelt G, Dekker T (2019) The prognostic value of tumour–stroma ratio in primary breast cancer with special attention to triple-negative tumours: a review. Breast Cancer Res Treat 173:55–64
de Kruijf EM, van Nes JGH, van de Velde CJH, Putter H (2011) Smit VTHBM, Liefers GJ, Kuppen PJK, Tollenaar RAEM, Mesker WE: Tumor–stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 125(3):687–696
Guedj N, Blaise L, Cauchy F, Albuquerque M, Soubrane O, Paradis V (2021) Prognostic value of desmoplastic stroma in intrahepatic cholangiocarcinoma. Mod Pathol 34(2):408–416
Cao L, Sun P-L, He Y, Yao M, Gao H (2020) Desmoplastic reaction and tumor budding in cervical squamous cell carcinoma are prognostic factors for distant metastasis: a retrospective study. Cancer Manag Res 12:137–144
Kemi NA, Eskuri M, Pohjanen V-M, Karttunen TJ, Kauppila JH (2019) Histological assessment of stromal maturity as a prognostic factor in surgically treated gastric adenocarcinoma. Histopathology 75(6):882–889
Hacking SM, Chakraborty B, Nasim R, Vitkovski T, Thomas R (2021) A holistic appraisal of stromal differentiation in colorectal cancer: biology, histopathology, computation, and genomics. Pathology - Research and Practice 220:153378
Ueno H, Ishiguro M, Nakatani E, Ishikawa T, Uetake H, Murotani K, Matsui S, Teramukai S, Sugai T, Ajioka Y et al: Prognostic value of desmoplastic reaction characterisation in stage II colon cancer: prospective validation in a Phase 3 study (SACURA Trial). British Journal of Cancer 2021.
Hacking SM, Wu D, Alexis C, Nasim M (2022) A novel superpixel approach to the tumoral microenvironment in colorectal cancer. Journal of Pathology Informatics 13:100009
Millar EK, Browne LH, Beretov J, Lee K, Lynch J, Swarbrick A, Graham PH (2020) Tumour stroma ratio assessment using digital image analysis predicts survival in triple negative and luminal breast cancer. Cancers (Basel) 12:12
Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. Arch Pathol Lab Med 134(6):907–922
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142(11):1364–1382
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ, Coleman HG et al (2017) QuPath: Open source software for digital pathology image analysis. Sci Rep 7(1):16878
R. Salgado CD, S. Demaria, N. Sirtaine, F. Klauschen, G. Pruneri, S.Wienert, G. Van den Eynden, F. L. Baehner, F. Penault-Llorca, E. A. Perez, E. A. Thompson, W. F. Symmans, A. L. Richardson, J. Brock, C. Criscitiello, H. Bailey, M. Ignatiadis, G. Floris, J. Sparano, Z. Kos, T. Nielsen, D. L. Rimm, K. H. Allison, J. S. Reis-Filho, S. Loibl, C. Sotiriou, G. Viale, S. Badve, S. Adams, K. Willard-Gallo, S. Loi 2014 The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Annals of Oncology, 26.
Kurozumi SMH, Kurosumi M, Inoue K, Fujii T, Horiguchi J, Shirabe K, Oyama T, Kuwano H (2019) Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncol Letters 17(2647):2656
Mesker WJJ, Szuhai K, Heer P, Morreau H, Tanke H, Tollenaar R (2007) The carcinoma stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cellular Oncology 29(2007):387–398
van Pelt GW, Sandberg TP, Morreau H, Gelderblom H, van Krieken J, Tollenaar R, Mesker WE (2018) The tumour-stroma ratio in colon cancer: the biological role and its prognostic impact. Histopathology 73(2):197–206
van Pelt GKJ, Lips I, Peters F, van Klaveren D, Boonstra J, de Steur W, Tollenaar RSA, Mesker W, Slingerland M (2020) The value of tumor-stroma ratio as predictor of pathologic response after neoadjuvant chemoradiotherapy in esophageal cancer. Clinical and Transliational Radiation Oncology 20(39):44
Moorman A VR, Heijmans H, van der Palen J, Kouwenhoven E 2012 The prognostic value of tumour-stroma ratio in triple-negative breast cancer. Eur J Surg Oncol.
Yanai H Yk, Ishida M, Tsuta K, Sekimoto M, Sugie T 2021 Presence of myxoid stromal change and fibrotic focus in pathological examination are prognostic factors of triple-negative breast cancer: Results from a retrospective single-center study. Plos One.
Zhai QFJ, Lin Q, Liu X, Li J, Hong R, Wang S (2019) Tumor stromal type is associated with stromal PD-L1 expression and predicts outcomes in breast cancer. Plos One 14(10):e0223325
Li B PG, Yao J, Ding Q, Jia P, Zhao Z 2021 Cell-type deconvolution analysis identifies cancer-associated myofibroblast component as a poor prognostic factor in multiple cancer types. Oncogene, 40.
Winslow SLK, Edsjo A, Larsson C (2016) The expression pattern of matrix-producing tumor stroma is of prognostic importance in breast cancer. BMC Cancer 16:841
Stanton SE, Adams S, Disis ML (2016) Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2(10):1354–1360
Ahn SCY, Soo A, Kim M, Woo J, Park S (2020) Changes and prognostic values of tumor- infiltrating lymphocyte subsets after primary systemic therapy in breast cancer. PLoS ONE 15(5):e0233037
Kos Z (2020) Pitfalls in assessing stromal tumor infiltrating lymphocytes (sTILs) in breast cancer. npj Breast Cancer 6:17
Sarker IH (2021) Machine learning: algorithms, real-world applications and research directions. SN Computer Science 2(3):160
Breiman L (2001) Random Forests. Mach Learn 45(1):5–32
Berardi VL, Zhang GP (2003) An empirical investigation of bias and variance in time series forecasting: modeling considerations and error evaluation. IEEE Trans Neural Netw 14(3):668–679
Wolpert D (1997) On bias plus variance. Neural Comput 9:1211–1243
Geman S, Bienenstock E, Doursat R (1992) Neural networks and the bias/variance dilemma. Neural Comput 4:1–58
Berardi V, Zhang P (2003) An empirical investigation of bias and variance in time series forecasting: modeling considerations and error evaluation IEEE transactions on neural networks / a publication of the IEEE. Neural Networks Council 14:668–679
Shi C, Pun C-M (2018) Superpixel-based 3D deep neural networks for hyperspectral image classification. Pattern Recogn 74:600–616
Acknowledgements
We thank Alexander Perry and Kathy Quinn for their role as research coordinators. Figures were constructed at Biorender.com.
Author information
Authors and Affiliations
Contributions
M. N., D. W., and S. H. developed the theoretical formalism. D. W., S. H., and M. N. contributed to the acquisition of data. D. W. performed the analytic calculations and performed the numerical simulations. D. W., S. H., H. C., M. A., and M. N. contributed to the final version of the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, D., Hacking, S.M., Chavarria, H. et al. Computational portraits of the tumoral microenvironment in human breast cancer. Virchows Arch 481, 367–385 (2022). https://doi.org/10.1007/s00428-022-03376-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03376-7