Abstract
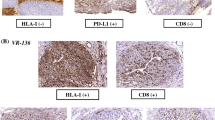
Immunotherapeutic strategies are increasingly used in the treatment of a number of malignancies including high-grade urothelial carcinoma (HGUC) of the bladder. Because of this, detailed and accurate assessment of the tumor immune microenvironment is paramount. In this study, we aimed to correlate the composition of the tumor immune microenvironment with oncologic outcome and the expression of two cancer testis antigens (CTAs), CT10 and PRAME, potential cancer vaccine targets, as well as major histocompatibility complex I (MHC I), a molecule associated with tumor immune escape and resistance to immunotherapy. Triplicate tissue microarrays (TMAs) were constructed using 207 cases of HGUC of the bladder. Oncologic outcome data was gathered for each case. Consecutive sections from the TMA blocks were stained with CD3, CD4, CD8, FOXP3, PD1, PD-L1, CT10, PRAME, and MHC I. Twenty-one percent and 15% of cases expressed CT10 and PRAME, respectively. Eighty-eight percent of cases showed absent or decreased MHC I expression. CT10-expressing tumors showed a significantly worse disease specific survival (p = 0.007, hazard ratio 2.245, confidence interval 1.223–4.122). CT10, PRAME, and MHC I expression significantly correlated with other some immune parameters. CT10 and PRAME are expressed in a subset of HGUC and CTA and MHC I expression correlate with a number of important immune parameters. Together, these findings highlight the potential for exploring novel immune therapeutic strategies in HGUC. Additional studies evaluating the clinical relevance of these findings are warranted.




Similar content being viewed by others
References
Hodgson A, Liu SK, Vesprini D, Xu B, Downes MR (2018) Basal-subtype bladder tumours show a “hot” immunophenotype. Histopathology 73:748–757. https://doi.org/10.1111/his.13696
Hodgson A, Xu B, Satkunasivam R, Downes MR (2018) Tumour front inflammation and necrosis are independent prognostic predictors in high-grade urothelial carcinoma of the bladder. J Clin Pathol 71:154–160. https://doi.org/10.1136/jclinpath-2017-204562
Liu X, Dowell AC, Patel P, Viney RP, Foster MC, Porfiri E, James ND, Bryan RT (2014) Cytokines as effectors and predictors of responses in the treatment of bladder cancer by bacillus Calmette-Guérin. Future Oncol 10:1443–1456. https://doi.org/10.2217/fon.14.79
Zuiverloon TCM, Nieuweboer AJM, Vékony H, Kirkels WJ, Bangma CH, Zwarthoff EC (2012) Markers predicting response to bacillus Calmette-Guérin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol 61:128–145. https://doi.org/10.1016/j.eururo.2011.09.026
Horn T, Laus J, Seitz AK, Maurer T, Schmid SC, Wolf P, Haller B, Winkler M, Retz M, Nawroth R, Gschwend JE, Kübler HR, Slotta-Huspenina J (2016) The prognostic effect of tumour-infiltrating lymphocytic subpopulations in bladder cancer. World J Urol 34:181–187. https://doi.org/10.1007/s00345-015-1615-3
Winerdal ME, Marits P, Winerdal M, Hasan M, Rosenblatt R, Tolf A, Selling K, Sherif A, Winqvist O (2011) FOXP3 and survival in urinary bladder cancer. BJU Int 108:1672–1678. https://doi.org/10.1111/j.1464-410X.2010.10020.x
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562. https://doi.org/10.1038/nature13904
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387:1909–1920. https://doi.org/10.1016/S0140-6736(16)00561-4
Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, Arranz JÁ, Pal S, Ohyama C, Saci A, Qu X, Lambert A, Krishnan S, Azrilevich A, Galsky MD (2017) Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet 18:312–322. https://doi.org/10.1016/S1470-2045(17)30065-7
Powles T, O’Donnell PH, Massard C et al (2017) Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 3:e172411. https://doi.org/10.1001/jamaoncol.2017.2411
Donin NM, Lenis AT, Holden S, Drakaki A, Pantuck A, Belldegrun A, Chamie K (2017) Immunotherapy for the treatment of urothelial carcinoma. J Urol 197:14–22. https://doi.org/10.1016/j.juro.2016.02.3005
Muthigi A, George AK, Brancato SJ, Agarwal PK (2016) Novel immunotherapeutic approaches to the treatment of urothelial carcinoma. Ther Adv Urol 8:203–214. https://doi.org/10.1177/1756287216628784
Guo C, Manjili MH, Subjeck JR et al (2013) Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res 119:421–475. https://doi.org/10.1016/B978-0-12-407190-2.00007-1
Wong KK, Li WA, Mooney DJ, Dranoff G (2016) Advances in therapeutic cancer vaccines. Adv Immunol 130:191–249. https://doi.org/10.1016/bs.ai.2015.12.001
Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C, Faulkner I, Mellstedt H (2014) Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol 11:509–524. https://doi.org/10.1038/nrclinonc.2014.111
Boon T, Coulie PG, Van den Eynde B (1997) Tumor antigens recognized by T cells. Immunol Today 18:267–268. https://doi.org/10.1016/S0167-5699(97)80020-5
Whitehurst AW (2014) Cause and consequence of cancer/testis antigen activation in cancer. Annu Rev Pharmacol Toxicol 54:251–272. https://doi.org/10.1146/annurev-pharmtox-011112-140326
Gjerstorff MF, Andersen MH, Ditzel HJ (2015) Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget 6:15772–15787. https://doi.org/10.18632/oncotarget.4694
Yin B, Liu G, Wang X-S, Zhang H, Song YS, Wu B (2012) Expression profile of cancer-testis genes in transitional cell carcinoma of the bladder. Urol Oncol 30:886–892. https://doi.org/10.1016/j.urolonc.2010.08.017
Sharma P, Gnjatic S, Jungbluth AA et al (2003) Frequency of NY-ESO-1 and LAGE-1 expression in bladder cancer and evidence of a new NY-ESO-1 T-cell epitope in a patient with bladder cancer. Cancer Immun 3:19
Lausenmeyer EM, Braun K, Breyer J, Gierth M, Denzinger S, Burger M, Voelker HU, Otto W (2018) Strong expression of cancertestis antigens CTAG1B and MAGEA3 is correlated with unfavourable histopathological features and MAGEA3 is associated with worse progression-free survival in urothelial bladder cancer. Urol Int 102:1–6. https://doi.org/10.1159/000493577
Sharma P, Shen Y, Wen S, Bajorin DF, Reuter VE, Old LJ, Jungbluth AA (2006) Cancer-testis antigens: expression and correlation with survival in human urothelial carcinoma. Clin Cancer Res 12:5442–5447. https://doi.org/10.1158/1078-0432.CCR-06-0527
Epping MT, Wang L, Edel MJ, Carlée L, Hernandez M, Bernards R (2005) The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell 122:835–847. https://doi.org/10.1016/j.cell.2005.07.003
van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJM (2016) Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 16:219–233. https://doi.org/10.1038/nrc.2016.16
Tagliamonte M, Petrizzo A, Tornesello ML, Buonaguro FM, Buonaguro L (2014) Antigen-specific vaccines for cancer treatment. Hum Vaccines Immunother 10:3332–3346. https://doi.org/10.4161/21645515.2014.973317
Garrido F, Aptsiauri N, Doorduijn EM, Garcia Lora AM, van Hall T (2016) The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr Opin Immunol 39:44–51. https://doi.org/10.1016/j.coi.2015.12.007
Hodgson A, Xu B, Downes MR (2017) p53 immunohistochemistry in high-grade urothelial carcinoma of the bladder is prognostically significant. Histopathology 71:296–304. https://doi.org/10.1111/his.13225
Zhuang R, Zhu Y, Fang L et al (2006) Generation of monoclonal antibodies to cancer/testis (CT) antigen CT10/MAGE-C2. Cancer Immun 6:7
Chevalier MF, Nardelli-Haefliger D, Domingos-Pereira S, Jichlinski P, Derré L (2014) Immunotherapeutic strategies for bladder cancer. Hum Vaccines Immunother 10:977–981. https://doi.org/10.4161/hv.27621
Gupta M, Kates M, Bivalacqua TJ (2019) Immunotherapy in nonmuscle invasive bladder cancer: current and emerging treatments. Curr Opin Oncol 31:183–187. https://doi.org/10.1097/CCO.0000000000000533
Dutcher GMA, Bilen MA (2018) Therapeutic vaccines for genitourinary malignancies. Vaccines 6:E55. https://doi.org/10.3390/vaccines6030055
Jin S, Cao S, Li J, Meng Q, Wang C, Yao L, Lang Y, Cao J, Shen J, Pan B, Hu J, Yu Y (2018) Cancer/testis antigens (CTAs) expression in resected lung cancer. OncoTargets Ther 11:4491–4499. https://doi.org/10.2147/OTT.S159491
Futawatari N, Fukuyama T, Yamamura R, Shida A, Takahashi Y, Nishi Y, Ichiki Y, Kobayashi N, Yamazaki H, Watanabe M (2017) Early gastric cancer frequently has high expression of KK-LC-1, a cancer-testis antigen. World J Gastroenterol 23:8200–8206. https://doi.org/10.3748/wjg.v23.i46.8200
Grah JJ, Katalinic D, Juretic A, Santek F, Samarzija M (2014) Clinical significance of immunohistochemical expression of cancer/testis tumor-associated antigens (MAGE-A1, MAGE-A3/4, NY-ESO-1) in patients with non-small cell lung cancer. Tumori 100:60–68. https://doi.org/10.1700/1430.15817
Yoshida N, Abe H, Ohkuri T et al (2006) Expression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens and T cell infiltration in non-small cell lung carcinoma and their prognostic significance. Int J Oncol 28:1089–1098
Matković B, Juretić A, Spagnoli GC, Šeparović V, Gamulin M, Šeparović R, Šarić N, Bašić-Koretić M, Novosel I, Krušlin B (2011) Expression of MAGE-A and NY-ESO-1 cancer/testis antigens in medullary breast cancer: retrospective immunohistochemical study. Croat Med J 52:171–177
Chitale DA, Jungbluth AA, Marshall DS, Leitao MM, Hedvat CV, Kolb D, Spagnoli GC, Iversen K, Soslow RA (2005) Expression of cancer-testis antigens in endometrial carcinomas using a tissue microarray. Mod Pathol Off J U S Can Acad Pathol Inc 18:119–126. https://doi.org/10.1038/modpathol.3800232
Prasad ML, Jungbluth AA, Patel SG, Iversen K, Hoshaw-Woodard S, Busam KJ (2004) Expression and significance of cancer testis antigens in primary mucosal melanoma of the head and neck. Head Neck 26:1053–1057. https://doi.org/10.1002/hed.20112
Bandić D, Juretić A, Sarcević B et al (2006) Expression and possible prognostic role of MAGE-A4, NY-ESO-1, and HER-2 antigens in women with relapsing invasive ductal breast cancer: retrospective immunohistochemical study. Croat Med J 47:32–41
Zhang Y, Zhang Y, Zhang L (2019) Expression of cancer-testis antigens in esophageal cancer and their progress in immunotherapy. J Cancer Res Clin Oncol 145:281–291. https://doi.org/10.1007/s00432-019-02840-3
Nishiyama T, Tachibana M, Horiguchi Y et al (2001) Immunotherapy of bladder cancer using autologous dendritic cells pulsed with human lymphocyte antigen-A24-specific MAGE-3 peptide. Clin Cancer Res 7:23–31
Obara W, Ohsawa R, Kanehira M, Takata R, Tsunoda T, Yoshida K, Takeda K, Katagiri T, Nakamura Y, Fujioka T (2012) Cancer peptide vaccine therapy developed from oncoantigens identified through genome-wide expression profile analysis for bladder cancer. Jpn J Clin Oncol 42:591–600. https://doi.org/10.1093/jjco/hys069
Colombel M, Heidenreich A, Martínez-Piñeiro L, Babjuk M, Korneyev I, Surcel C, Yakovlev P, Colombo R, Radziszewski P, Witjes F, Schipper R, Mulders P, Witjes WPJ (2014) Perioperative chemotherapy in muscle-invasive bladder cancer: overview and the unmet clinical need for alternative adjuvant therapy as studied in the MAGNOLIA trial. Eur Urol 65:509–511. https://doi.org/10.1016/j.eururo.2013.10.056
Sabbatino F, Schwab JH, Ferrone S, Ferrone CR (2013) Evolution of studies of HLA class I antigen processing machinery (APM) components in malignant cells. Clin Transpl 453–463
Garrido F, Ruiz-Cabello F, Aptsiauri N (2017) Rejection versus escape: the tumor MHC dilemma. Cancer Immunol Immunother 66:259–271. https://doi.org/10.1007/s00262-016-1947-x
Acknowledgments
CT10, PRAME, and MHC I immunohistochemical staining was performed in the Department of Pathology, Memorial Sloan Kettering Cancer Center in New York, NY, USA. The SP263 antibody was purchased using a Sunnybrook Health Sciences Centre departmental Educational Grant courtesy of Astra Zeneca.
Author information
Authors and Affiliations
Contributions
Drs. Downes and Xu conceived and designed the study. Drs. Hodgson, Xu, and Downes gathered the data. Drs. Hodgson, Katabi, Jungbluth, Xu, and Downes reviewed and interpreted the data. Dr. Xu performed the statistical analysis. Drs. Hodgson, Katabi, Jungbluth, Xu, and Downes contributed to drafting and review of the manuscript as well as approval of the final version. Dr. Downes takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
This project was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre in Toronto, Ontario, Canada (REB 187-2016).
Conflict of interest
Dr. Downes has received compensation from Hoffman – La Roche and Astra Zeneca for participating on advisory boards and honoraria from Astra Zeneca.
Dr. Xu has received honoraria from Merck.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hodgson, A., Jungbluth, A.A., Katabi, N. et al. Evaluation of cancer testis antigen (CT10, PRAME) and MHC I expression in high-grade urothelial carcinoma of the bladder. Virchows Arch 476, 535–542 (2020). https://doi.org/10.1007/s00428-019-02661-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02661-2