Abstract
The tritocerebral commissure giant (TCG) of the grasshopper Schistocerca gregaria is one of the best anatomically and physiologically described arthropod brain neurons. A member of the so-called Ventral Giant cluster of cells, it integrates sensory information from visual, antennal and hair receptors, and synapses with thoracic motor neurons in order to initiate and regulate flight behavior. Its ontogeny, however, remains unclear. In this study, we use bromodeoxyuridine incorporation and cyclin labeling to reveal proliferative neuroblasts in the region of the embryonic brain where the ventral giant cluster is located. Engrailed labeling confirms the deutocerebral identity of this cluster. Comparison of soma locations and initial neurite projections into tracts of the striate deutocerebrum help identify the cells of the ventral cluster in both the embryonic and adult brain. Reconstructions of embryonic cell lineages suggest deutocerebral NB1 as being the putative neuroblast of origin. Intracellular dye injection coupled with immunolabeling against neuron-specific horseradish peroxidase is used to identify the VG1 (TCG) and VG3 neurons from the ventral cluster in embryonic brain slices. Dye injection and backfilling are used to document axogenesis and the progressive expansion of the dendritic arbor of the TCG from mid-embryogenesis up to hatching. Comparative maps of embryonic neuroblasts from several orthopteroid insects suggest equivalent deutocerebral neuroblasts from which the homologous TCG neurons already identified in the adult brain could originate. Our data offer the prospect of identifying further lineage-related neurons from the cluster and so understand a brain connectome from both a developmental and evolutionary perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The “identified neuron” concept has contributed significantly to our understanding of the neuronal basis of insect behavior (see Huber and Markl 2012). In the grasshopper ventral nerve cord, for example, various identified interneurons integrating sensory information from the cerci, auditory organs, wing-hinge, and leg joints, have been shown to evoke and regulate a range of motor behaviors such as walking, jumping, flight, and escape (see Burrows 1996). In the brain, identified neurons integrating information from sensory organs such as the compound eyes, ocelli, antennae, or head hairs (Rowell 1971; O'Shea and Williams 1974; O'Shea et al. 1974; Simmons 1980) have been demonstrated to mediate optomotor activity: for deviation detection in flight (Griss and Rowell 1986; Hensler 1990; Hensler and Rowell 1990), in visual collision avoidance (Simmons and Rind 1992, 1997; Rind and Bramwell 1996; Santer et al. 2006; Rind et al. 2008), in walking (Kien and Williams 1983), and in stridulatory and reproductive behavior (Hedwig and Heinrich 1997; Hedwig 2000).
Paralleling these descriptions of identified neurons in adult grasshoppers, the advent of neuroblast maps for the ventral nerve cord (Bate 1976; Bate and Grunewald 1981; Doe and Goodman 1985) has meant that lineage analyses can be employed to reveal serial and even interspecific homologies for identified neurons such as the so-called G neuron in the VNC of grasshopper, Drosophila and Manduca, and thus provide new insights into the evolutionary relationships between insect nervous systems (see Thomas et al. 1984; Pearson et al. 1985; Boyan and Ball 1993; Boyan and Williams 1995). Developmental studies have documented the embryonic and postembryonic development of at least one identified neuron (DCMD; Bentley and Toroian-Raymond 1981) of the grasshopper brain, but in predating any neuroblast map for reference, no lineage analysis could be undertaken at that time. Indeed to date, not a single identified brain neuron has been traced to its neuroblast of origin in any insect.
One of the best anatomically and physiologically described invertebrate brain neurons is the tritocerebral commissure giant (TCG) of the grasshopper which integrates sensory information from a range of visual, antennal, and hair receptors (Bacon and Tyrer, 1978) in order to initiate and regulate flight behavior (Bacon and Möhl 1979, 1983; Bicker and Pearson 1983; Möhl and Bacon 1983; Kutsch and Hemmer 1994). The TCG is a member of the ventral giant (VG) cluster of cells first identified by Williams (1972, 1975) and located in the so-called crotch region of the deutocerebrum. In this study, we document the embryonic development of the TCG and take advantage of the fact that a complete neuroblast map is available for the brain of the grasshopper (Zacharias et al. 1993; Williams et al. 2005) in order to identify its lineage of origin.
Given that neuroblast maps of the brain are now available for a range of insects (see Urbach and Technau 2003) including some in which putatively homologous neurons to the TCG of the grasshopper have been identified (Bacon 1980), the data from our study offer the prospect of identifying conserved developmental programs generating such neurons and therefore understanding brain circuitry from an evolutionary perspective.
Materials and methods
Animals
Schistocerca gregaria were kept in crowded cultures at the Universities of Konstanz, Munich, and Sussex. Eggs were incubated at 30 °C in the day and 25 °C in the night in moist aerated containers (relative humidity approximately 60%) according to a 12/12 h light/dark regime. Embryos were staged at time intervals equal to percentage of embryogenesis as described by Bentley et al. (1979). The results described below were collated from experiments on over 80 animals. All experiments were performed according to the guidelines for animal welfare as laid down by the Deutsche Forschungsgemeinschaft.
Immunolabeling
Embryos were dissected out of the egg, staged, washed in PBS (2 mM NaH2PO4, 15 mM Na2HPO4, 175 mM NaCl, pH 7.4), and fixed in Pipes-FA (100 mM Pipes, 2 mM EGTA, 1 mM MgSO4, 3.7% formaldehyde, pH 7.0) for 45–60 min. Preincubation was for 45 min in a solution of 0.1% PBT (PBS plus Triton X-100), 5% normal goat serum (NGS, Sigma), and 0.1% bovine serum albumin (BSA, Sigma) (pH 7.4).
Engrailed
Following preincubation as above, embryos were transferred to anti-Engrailed primary antibody (Mab 4D9, gift of E. Ball) diluted 1:1 in the preincubation solution for 16 h at room temperature (RT). After washing in PBT, embryos were incubated with Dako EnVision™ peroxidase (PO)-conjugated secondary antibody (GAM goat anti-mouse, 1:4 dilution) for 4 h at RT (see Boyan and Williams 2002 for further details).
Tubulin
α-Tubulin is one of the two globular proteins making up the microtubules of the eukaryotic cytoskeleton and has previously been used to trace neuronal processes in the grasshopper nervous system (Kononenko and Pflüger 2007; Ehrhardt et al. 2015). The protocol we employed here was exactly as described in Ehrhardt et al. (2015).
Proliferating cell nuclear antigen
Proliferating cell nuclear antigen, also known as cyclin, is an evolutionarily highly conserved 36-kD acidic nuclear protein associated with the cell cycle (Bravo and Celis 1980; Celis et al. 1987; Hall et al. 1990; Zudaire et al. 2004). The protein functions as a co-factor for DNA polymerase δ, therefore being an essential requirement for chromosome replication prior to mitosis (Bravo and Macdonald-Bravo, 1987; Shreeram and Blow, 2003). Immunocytochemical detection of proliferating cell nuclear antigen (PCNA) has therefore been extensively used to assess the cell proliferation status of insect cells (e.g., Ng et al. 1990; Yamaguchi et al. 1991, 1995; Zudaire et al. 2004). Prior to PCNA antibody incubation, the preparations were placed in HIER buffer (19.0 ml 01 M citric acid, 81.0 ml of 0.1 M sodium citrate, pH 6.0) and heat-shocked at 80 °C for 20 min. The PCNA primary antibody (mouse, Dako Cytomation) was diluted 1:500 in a preincubating medium comprising 5% bovine serum albumin (BSA), 0.5% Triton X-100, 0.1% saponin in 0.1 M PBS (pH 7.4). After thorough washing in 0.1 M PBS, preparations were placed in preincubation medium to which the secondary antibody (GAM-Cy3, Sigma, 1:150 dilution) was added for 24 h at 4 °C in the dark and then processed as described in Boyan and Liu (2014).
Horseradish peroxidase
In insects, the horseradish peroxidase (HRP) epitope is neuron-specific (Jan and Jan 1982) and is not expressed by progenitor cells such as neuroblasts and ganglion mother cells. Preparations were exposed to the anti-HRP primary antibody (Jackson ImmunoResearch) diluted 1:200 in preincubating medium for 24 h at 4 °C. After washing, preparations were transferred to fresh incubation medium containing the secondary antibody (GAM-Cy3, Dianova, 1:150 dilution) and processed as for α-tubulin.
Specificity of each secondary antibody was confirmed by a lack of a specific staining pattern in the absence of the primary label.
Backfilling
Embryos were dissected out of the egg, staged, and washed in PBS at RT. For embryos older than 60%, legs, antennae, and mouthparts were removed and a dorsal incision made to remove the gut and yolk. The cuticle of the head was removed between the eye and the mandibular muscles. Embryos were fixed with minuten pins onto sylgard-coated petri dishes. Briefly, a circumesophageal connective between the subesophageal and prothoracic ganglia was cut and the stump leading to the brain was placed in a small vaseline well filled with a solution of 1.5–3% NiCl2 in distilled water. Perfusion was for 4 h at RT. Further processing, including silver intensification, followed the method of Bacon and Altman (1977). Selected preparations were mounted in Canada Balsam (Merck) for eventual embedding and sectioning.
For adults, after anesthesia by cold, one or both neck connectives of the intact animal were severed and the proximal cut ends were enclosed in situ in a vaseline cup containing a 1.5–5% solution of CoCl2. Backfilling was continued for 5–7 h at room temperature. The brain was then dissected from the preparation and processed using the whole mount method of Bacon and Altman (1977). The intensified tissue was embedded in soft Epon 812, sectioned at 40 μm and mounted in Permount.
Intracellular dye injection
Brain slices
Staged embryos were dissected out of the egg into ice-cold 0.1 M PBS and freed from embryonic membranes. Prior to embedding, brains were placed in a 1% methylene blue solution for 5 min to facilitate visual orientation of the brain. Brains were shock frozen, unfixed, at − 20 °C in 2-methylbutane, without sucrose and sectioned frontally (horizontally with respect to the body axis) on a Leica CM3050S cryostat at 16 μm thickness. Frozen sections were transferred onto frozen Superfrost® Plus (Menzel Glaeser) microscope slides, one slice per slide, and stored at − 18 °C. Intracellular injection with Alexa® 568 followed the protocol described in Boyan et al. (2012). Neuronal identity of impaled cells was subsequently confirmed via HRP-labeling using the protocol described above.
Whole mounts
Intracellular penetrations of somata in the brain at > 50% of embryogenesis required prior mechanical desheathing of the brain to prevent electrode damage. Dye filling with Lucifer Yellow (LY) was performed under a Zeiss compound microscope as for sections (see above), but with a × 40 water immersion lens.
The intracellular LY fills were fixed with 4% PFA for 45 min before washing and blocking in preincubation medium (see above). For anti-LY/anti-HRP double-labeling, the anti-LY was diluted 1:1000 in medium, the anti-HRP 1:250, and incubation was overnight in the dark at RT. After washing for 1 h in PBS (3–6 changes) preparations were incubated for 4 h in peroxidase-conjugated goat anti-rabbit secondary antibody (GAR-PO, Jackson ImmunoResearch, 1:250 dilution), washed, and counterstained with DAB using standard protocols.
Histology
Osmium tetroxide-ethyl gallate
Staged embryos were dissected out of the egg into PBS, freed from membranes, placed in Carnoy fixative (60% ethanol, 30% chloroform, 10% glacial acetic acid) for 1 h, and then processed as described in Boyan et al. (1993).
Bielschowsky’s reduced silver method
This staining method reveals mainly axonal membranes and/or neurofilaments. The protocol used was exactly as described in Boyan et al. (1993).
Bromodeoxyuridine incorporation
5-bromodeoxyuridine (BrdU) incorporation in whole embryo culture was undertaken to reveal proliferative neuroblasts in the DC. Progeny inherit the label and so partial lineages can also be revealed. The protocol for this and the subsequent BrdU immunolabeling was exactly as previously described in Williams et al. (2005). On completion, preparations were embedded in Epon and sectioned at 20 μm thickness.
Imaging and reconstructions
Optical sections of brain slice preparations were acquired with a Leica TCS SP5 confocal laser-scanning microscope. Z-stacks of confocal images were processed using public domain software (ImageJ) and only the contrast and resolution subsequently altered. Histological sections were viewed on a Zeiss Axioskop2 equipped with differential interference contrast (DIC) optics and images captured with a 1.3 MP color CCD camera (Scion Corp.) via Scion VisicaptureTM software. Camera-lucida drawings of neurons were made using a drawing tube attached to a Zeiss compound microscope at × 400 magnification.
Terminology
Neuroarchitecture is described with respect to the neuraxis and not the body axis. The front of the brain (in the head) is defined as being neurally ventral, the top of the brain as neurally anterior, the back of the brain as neurally dorsal, and the base of the brain as neurally posterior. Planes of section are defined such that horizontal to the neuroaxis means parallel to the neuroaxis (anterior is to the top). Location within lineages accords to age so that cells closer to the neuroblast are younger, and those distant to the neuroblast are older.
Results
The ventral giant cluster of neurons
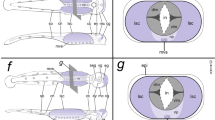
The somata of the ventral giant (VG) neurons 1–3 (nomenclature of Williams 1972, 1975) are the largest members of a cluster of cells located bilaterally of the midline in what is anatomically considered to be the deutocerebrum of the adult grasshopper brain (Fig. 1a; see Bacon and Tyrer 1978). However, molecular confirmation of this segmental identity has been lacking to date (but see below). Reconstructions from histology show that the VG1 (TCG) and VG2 cells each direct initial neurites and dendritic processes into the striate neuropil of the dorsal DC as well as towards sensory neuropils of the protocerebrum (Fig. 1b). In each case, a descending axon projects ipsilaterally into the circumesophageal connective (coc) to the subesophageal ganglion (SOG) and then to the ventral nerve cord (Fig. 1b, c; see Williams 1975; Bacon and Tyrer 1978). The VG3 neuron, by contrast, directs an initial neurite anteriorly and then contralaterally via commissure DC IX (see Boyan et al. 1993) from which dendritic process projects laterally towards protocerebral neuropils. Its axon descends in the contralateral coc to the ventral nerve cord (Fig. 1b). Intracellular cobalt injection into the VG1 (TCG) neuron (Fig. 1c) confirms the major morphological features revealed by histology, notably, the characteristic contralateral axonal projection to the subesophageal ganglion (SOG) via the minor branch of the tritocerebral commissure (TCC = PC XXIV of Boyan et al. 1993).
The ventral giant (VG) cluster of neurons in the brain of the adult grasshopper Schistocerca gregaria. a Semi-schematic drawing of the brain of the adult grasshopper viewed frontally (from ventral) shows the somata of the VG neurons 1–3 located bilaterally on either side of the midline in the posterior deutocerebrum (DC). b Reconstruction of the bilateral VG cluster of cells in the region of the dashed rectangle in a, as revealed by serial sections following osmium staining. The VG1 (TCG) and VG2 cells each direct initial neurites posteriorly from which dendritic processes project both laterally into the striate neuropil of the DC as well as anteriorly towards sensory neuropils of the protocerebrum (PC). An axon then descends ipsilaterally into the circumesophageal connective (coc) to the ventral nerve cord (not shown). The VG3 neurons direct an initial neurite contralaterally via commissure DC IX (not shown but see Boyan et al. 1993) from which dendritic process project anteriorly towards protocerebral neuropils. An axon descends in the contralateral coc to the ventral nerve cord (not shown). c Camera lucida drawing of a VG1 (TCG) neuron following intracellular staining with cobalt in a whole mount preparation confirms the major neuro-architectural features revealed from histology in panel b. Note the characteristic axon projection ipsilaterally into the tritocerebral commissure (TCC) and then via the minor branch (open arrow) into the contralateral coc to the subesophageal ganglion (SOG). The cocs are drawn shortened. Arrow points to anterior (a) and applies to all panels. Other abbreviations: AL antennal lobe, OL optic lobe, TC tritocerebrum. Scale bar represents 300 μm in panel a; 150 μm in panel b; 240 μm in panel c. Panels a, b modified from Williams (1972)
The stereotypic soma locations and initial neurite projections of the VG1 (TCG), VG2, and VG3 neurons provide morphological criteria with which to locate this cell cluster in the adult brain (Figs. 1 and 2a). We are also able to identify a neuron typically located just posterior to the VG cluster, the neurite of which shares a tract with the VG1 and VG2 cells (Fig. 2a (ii)). These same criteria enable us to recognize this neuron near mid-embryogenesis (Fig. 2b) and thereby locate the VG cluster in the embryonic brain. The soma locations and neurite projections of embryonic cells from this cluster as reconstructed from serial histological sections following osmium staining (Fig. 2b (i)), and by immunolabeling against neuron-specific HRP (Fig. 2b (ii, iii)), are consistent with their adult counterparts (Fig. 2a (ii)) and allow us to identify the individual embryonic VG1, VG2, and VG3 cells.
Stereotypic soma locations and neurite projections into tracts of the DC identify cells of the VG cluster (dark gray) in the adult (a) and embryonic (47%, b) brain. Reconstructions in each case are from serial histological sections following osmium staining. Arrow indicates anterior throughout. A further cell (light gray, black star) posterior to the VG group is identifiable in both the adult (a, ii) and embryo (b, i) and projects into a common tract with the VG1 and VG2 cells. Neurite projections are shown abbreviated. a (i) is a photomicrograph of a frontal section through the brain following Bielschowsky histology showing somata of the VG1 (TCG), VG2, and VG3 neurons. b (ii, iii) are confocal images from brain slices following labeling against neuron-specific HRP (green). b (ii) shows the putative VG3 cell with an axon (white arrowhead) projecting anteriorly towards the brain midline. b (iii) shows the putative VG2 cell extending a process (open white arrowhead) posteriorly towards a tract (white arrowhead) it shares with a more posterior neuron (white star) in both the adult and embryonic brain (black star in a, ii and b, i). Other abbreviation: fg frontal ganglion. Scale bar represents 125 μm in a (i), 50 μm in a (ii), 12 μm in b (i); 10 μm in b (ii, iii)
Segmental identity
As in all arthropods, engrailed expression delimits the posterior compartment of each segment in the embryonic grasshopper (see Patel et al. 1989a, b). Whereas the expression pattern in neuromeres of the VNC is iterative, that in the early embryonic brain is neuromere-specific (Fig. 3; see Boyan and Williams, 2002, Urbach and Technau 2003 for detailed analyses). Three neuromeres are present (PC, DC, TC) and the VG cluster is clearly located posterior to the protocerebral expression band and therefore within the deutocerebral neuromere of the brain (Fig. 3).
Deutocerebral identity of the VG cluster of cells confirmed by engrailed expression in the early embryo. Semi-schematics (not to scale) have been traced from horizontal (relative to the neuraxis) sections of embryos following engrailed (En) labeling at 28% (a), 34% (b), and 37% (c) of embryogenesis. En-positive cells (NBs and progeny) are shown black; En-negative cells gray. Posterior segmental borders are dashed red. The approximate location of the VG cluster of cells in the DC of the right brain hemisphere is indicated by a blue asterisk. En-positive cells occur as bands at the posterior borders of the mandibular (Ma), tritocerebral (TC), deutocerebral (DC), and protocerebral (PC) neuromeres. Anterior to the En-labeled cells in the PC are the secondary headspot cells (shs) which do not mark a segmental border but are the progeny of identified En-positive protocerebral neuroblasts (see Boyan and Williams 2002). Equivalent secondary headspot cells are found serially in each neuromere (black arrowheads). Arrow points to anterior (A) for all panels
Ontogeny of the ventral giant group
All neuroblasts (NBs) of the PC, DC, and TC of the grasshopper embryonic brain have been mapped (Fig. 4a; see Zacharias et al. 1993). Their close physical proximity and similar morphological properties (Figs. 1 and 2) are consistent with the cells of the VG cluster deriving from a common neuroblast, and their location in the so-called crotch region of the deutocerebrum (Fig. 3, and see Bacon and Tyrer 1978) suggests that this neuroblast is most likely located near the anterior-median edge of the DC. Indeed, an ontogeny from NBs of this deutocerebral region has previously been proposed (Müller 1995), but without a lineage analysis, the individual NB of origin could not be identified.
Proliferative neuroblasts (NBs) in the DC of the embryonic grasshopper brain. a Schematic (not to scale) maps the distribution of NBs in the PC, DC, and TC of the right brain hemisphere as seen at mid-embryogenesis. NBs occupy stereotypic locations and so can be individually identified (see Zacharias et al. 1993), but only those in the DC are labeled here. NBs 1, 6 (shaded black) of the DC have been proposed as being possible progenitors of the VG cluster of neurons (see Müller 1995). Path of the primary protocerebral commissure (PCC) is shaded gray. Midline (m) of the brain is indicated. Black arrow points to anterior (A) and applies to all panels. b–d. Photomicrographs (DIC) of brain slices from the DC at 32–35% of embryonic development following pulsed BrdU incorporation in whole embryo culture and subsequent anti-BrdU immunolabeling. NBs 1, 6, 7, and 11 are BrdU-positive (dark brown) and so are proliferative at these embryonic stages. BrdU has also been inherited by daughter progeny (white stars) thereby providing an estimate of the lineage in each brain slice (short white dashed lines). Edge of the brain is indicated by long dashed lines. e–g Confocal images of proliferative NBs in a brain slice from the DC of the brain at 50% of embryogenesis following double-immunolabeling against the cyclin PCNA (magenta) and the DNA label DAPI (blue). The optical stack analyzed is restricted to the NBs so the more ventral progeny are not captured. Cyclin labeling confirms that NBs 1, 6, 7, and 11 are in the S-phase of the cell cycle prior to mitosis. Other abbreviation: fg frontal ganglion. Scale bar represents 30 μm in b–d, 25 μm in e–g. Panel a modified from Boyan and Liu (2016)
In order to establish the ontogeny of the VG group, we first employed two separate markers for cell proliferation—BrdU incorporation in whole embryo culture and immunolabeling against a cyclin (PCNA) associated with DNA replication during the S-phase of the cell cycle (see the “Materials and methods” section). The cells of the VG cluster are already identifiable at 47% of embryogenesis (Fig. 2) so we focused on identifying those NBs of the anterior-median DCs that are mitotically active and generating lineages prior to this age. BrdU incorporation reveals that NBs 1, 6, 7, and 11 of the anterior-median DC are proliferative very early in embryogenesis (Fig. 4b–d). Since some of their progeny have also inherited this mitotic label, we can deduce the direction and extent of their initial lineages. NB1 lies most anteriorly in the DC, and its early progeny project in an anterior direction (Fig. 4c). However, as more cells are subsequently generated, the presence of the protocerebral commissure (PCC) further anterior (Fig. 4a) forces this lineage to be deflected posteriorly and then expand along the border of the DC with the stomodeum (Fig. 4d, and see Fig. 5). NB6 lies more posteriorly in the DC, and its early progeny appear to expand anteriorly and laterally to the lineage of NB1. Our second label, PCNA (cyclin), also identifies NB1, NB6, NB7, and NB11 in their stereotypic locations in the DC and demonstrates that these stem cells are still proliferative at 50% of embryogenesis (Fig. 4e–g).
Lineages of NB1 and NB6 in the DC of the brain at 57% of embryogenesis. a Photomicrographs (DIC) show partial lineages (short dashed white lines) of NBs 1 and 6 of the DC in three successively more dorsal brain sections (i–iii) viewed frontally following osmium ethyl gallate (OsEG) histology. Glia cells (black arrowheads) and their processes ensheath the brain and so delimit the DC from the frontal ganglion (fg). Other glial processes (white arrowheads) delimit lineages. Two large neurons (white star, white open circle) at the tip of the NB1 lineage have generated neurites (white/open arrowheads) directed anteriorly and laterally in the DC. These are characteristic for some members of the VG cluster. Arrow points to anterior (A) for panels a – e. b – e. Schematics (not to scale) from four different preparations following OsEG histology show the reconstructed lineages of NB1 (shaded green) and NB6 (shaded blue) based on consecutive frontal brain slices going respectively from ventral (i) to dorsal (ii, iii). Note the conserved appearance of the lineages in the respective preparations. Short black dashes indicate the approximate edge of the brain. Gray shading indicates a fiber tract of the DC known to contain axon projections from cells of the NB1 lineage (see also Fig. 5). White stars indicate cells in a location consistent with that of the VG group. f Photomicrograph (DIC) of a horizontal section of the DC at 47% of embryogenesis showing NB1 with its cap cell (c), a ganglion mother cell (GMC), and progeny (white stars). Arrow points to dorsal (D). Scale bar represents 20 μm for panel a, 15 μm for panel f
As the PCNA label is not inherited, we required additional means to visualize how the various lineages grow, and how they are spatially organized, in order to identify the individual stem cell with which the VG cluster is associated. Osmium staining reveals NBs 1 and 6 along with their respective lineages in the embryonic DC (Fig. 5a). The lineages are delimited by cellular processes similar to those which have previously been shown to express the glia-specific label Rega-1 in the brain (Boyan et al. 1995a). The lineages of NB1 and NB6, visualized at 57% of embryogenesis, have each retained the characteristic cellular architectures from earlier developmental stages (c.f. Fig. 4b–d). Closer inspection shows that two neighboring cells at the posterior tip of the lineage of NB1, thus representing early-born cells, generate initial neurites which project towards neuropilar tracts of the striate DC in a manner observed for VG1 and VG2 of the embryo and adult (c.f. Figs. 1 and 2).
Reconstructions of the respective lineages of NB1 and NB6 from serial sections at 57% (Fig. 5b–e) and 47% (Fig. 5f) of embryogenesis support the observations above based on proliferative labels. The lineages of NB1 and NB6 each comprise around 20 cells at mid-embryogenesis and possess a cellular neuroarchitecture which is consistent from preparation to preparation. The progeny of NB1 extend from the “crotch” region of the DC in a posterior direction along its border with the stomodeum thus encompassing the region containing the VG cluster (c.f. Fig. 2). The lineage of the more posterior NB6, by comparison, lies generally more dorsal and lateral to that of NB1. This is because the two lineages abut as they expand, resulting in the lineage of NB1 remaining more medial, whereas that of NB6 is deflected laterally. Such spatially induced morphogenetic realignment of lineages has been described elsewhere in the developing grasshopper brain (see Boyan et al. 1995b; Williams et al. 2005).
Lineage architecture in the DC leads us to propose that NB1 and not NB6 is the stem cell of origin for the VG cluster. Morphological data such as soma location (Fig. 5b (iii), c (iii), d (iii), e (ii)) and neurite projection patterns (Fig. 2, and see Fig. 6) suggest that the VG group comprises older, perhaps even first-born, cells of the lineage.
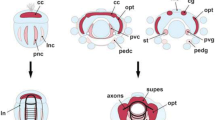
Intracellular dye injection and immunolabeling identify neuroblasts and progeny in the DC of the embryonic brain. Anterior is to the top unless otherwise specified. a Confocal image of a frontal brain slice at 58% of embryogenesis following successive intracellular injections (electrodes shown schematically) of Alexa® 568 dye (magenta) into identified neuroblasts NB1, NB6, and NB16 of the DC (see Fig. 4a for NB map) and subsequent immunolabeling against neuron-specific HRP (green). NBs are HRP-negative; neuronal somata and their projections in the neuropil of the protocerebrum (PC) are HRP-positive. b, c 3D confocal views of the DC (from panel a) at 58% of embryogenesis following intracellular injection (electrodes shown schematically) of Alexa® 568 dye (magenta) into NB1 and NB6 and subsequent immunolabeling against HRP (green). Dashed white line indicates border of brain. NB1 is visible outlined with white dashes anterior to NB6 in panel c. Other abbreviation: fg frontal ganglion. d Confocal image of a frontal brain slice at 55% of embryogenesis following intracellular injection (electrode shown schematically) of Alexa® 568 dye (magenta) into the putative TCG neuron of the DC and immunolabeling against neuron-specific HRP (green). e Higher power confocal image from panel d shows the putative TCG neuron (white cross) of the DC following intracellular injection of Alexa® 568 (magenta) and immunolabeling against neuron-specific HRP (green). Co-labeling is white (e.g., open white arrowheads). The initial axon segment of the stained neuron is visible (white arrowhead). Progeny of NB1 are visible anteriorly (white stars). Soma location, soma shape, and axon projection argue for the dye-filled neuron being the TCG. f 3D confocal image of the stained TCG neuron from panel e (soma indicated by white cross) but now rotated about its axis so as to reveal its neurite (white arrowhead) projecting dorsally and posteriorly towards the tritocerebrum (only Alexa® 568 is imaged here for clarity). Gray shading indicates the approximate ventral surface of the brain. g Confocal image of a brain section following intracellular injection of Alexa® 568 into NB1 of the DC. Subsequent immunolabeling reveals the HRP-positive VG3 neuron (white star) projecting a neurite (black arrowheads) anteriorly towards the brain midline (c.f. Fig. 2b). Scale bar represents 185 μm in panel a; 25 μm in panels b, c, e, f, g; 135 μm in panel d
Single cells of the ventral giant group
Anatomical characteristics which we have employed above to identify the VG cluster include relative soma locations and the projection pattern of initial neurites into neuropilar tracts (Fig. 2). As to lineage association, our reconstructions (Fig. 5) reveal embryonic progeny from the lineage of NB1 located at sites where, from the adult brain (c.f. Figs. 1b and 2a), we expect cells of the VG cluster to lie. None of the progeny of NB6 occupy locations which suggest similar associations.
To more rigorously identify individual cells of the VG group, and confirm their lineage association, we cut brain slices and then injected Alexa® 568 dye selectively into neuroblasts and their progeny. This enabled us to first identify candidate neuroblasts (Fig. 6a–c), and, when combined with immunolabeling against neuron-specific HRP, allowed us to identify their neuronal progeny (Fig. 6d–g). Successive intracellular injections of Alexa® 568 dye performed in the same preparation (Fig. 6a) reveal neuroblasts NB1, NB6, and NB16 in their stereotypic locations in the DC (c.f. Fig. 4a). The NBs are no longer dye-coupled to their progeny at this developmental stage (Fig. 6b, c), consistent with earlier observations from the VNC (Goodman and Spitzer 1979), so that lineages cannot be reconstructed directly by this method. Intracellular dye injection into a cell of the DC just after mid-embryogenesis (Fig. 6d, e) reveals an HRP-positive neuron with a soma located in the crotch region of the brain known to be occupied by members of the VG cluster (c.f. Figs. 1 and 2). Higher power confocal imaging (Fig. 6e) shows that the soma of this neuron lies at the tip of the lineage from NB1 and has a morphology stereotypic for the adult TCG (c.f. Fig. 1c; Bacon and Tyrer 1978). 3D-confocal imaging and subsequent rotation of the optical stack (Fig. 6f) reveals that the dye-filled cell extends an axon dorsally and posteriorly towards the TC, characteristic of the TCG (c.f. Fig. 1). In a further preparation (Fig. 6g), dye was injected into NB1 and subsequent immunolabeling reveals an adjacent HRP-positive neuron which extends a process anteriorly towards the brain midline as reported above for the VG3 neuron—the most anterior cell of the VG cluster (c.f. Fig. 2).
Embryonic development of the tritocerebral commissure giant
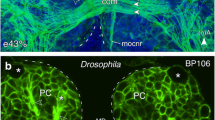
Immunolabeling against the cytoskeletal protein α-tubulin reveals the early axon scaffold in anterior neuromeres of the embryonic grasshopper nervous system (Fig. 7a). Two prominent commissures are situated anterior and posterior of the stomodeum in the protocerebrum (PCC) and tritocerebrum (TCC), respectively. Longitudinal fiber tracts link the brain with the mandibular (Ma) and maxillary (Mx) neuromeres of the future subesophageal ganglion via the circumesophageal connectives (coc). A defining morphological feature of the adult TCG neuron is its descending axonal projection via the TCC and its minor branch into the contralateral coc (see Fig. 1c). The TCG is one of only two neurons which projects into this minor branch, the other being the much smaller tritocerebral commissure dwarf (TCD; Tyrer et al. 1988). To map the development of the TCG neuron in the embryo, we first performed dye injections into the deutocerebral region of whole mount preparations where the somata of the VG cells are located. At 45% of embryogenesis, camera lucida reconstructions following such dye injections reveal axonal projections from deutocerebral cells in the coc, TCC, and VNC (Fig. 7b). The axon of the TCG can be seen in the minor branch of the TCC, with a growth cone extending contralaterally into the labial neuromere (La) of the future subesophageal ganglion (SOG). Reconstructions based on NiCl2 backfills from the VNC in whole mount preparations at different embryonic ages up to hatching (Fig. 7c) identify a cell which by mid-embryogenesis already possesses the morphology of soma shape and location, and dendritic projections into deutocerebral neuropil, which are characteristic of the adult TCG (c.f. Fig. 1c).
Axon scaffold of the early brain and morphology of the developing TCG neuron. a Confocal image following immunolabeling against α-tubulin shows the primary axon scaffold in anterior neuromeres (PC protocerebral, DC deutocerebral, TC tritocerebral, Ma mandibular, Mx maxillary) of the grasshopper central nervous system at 50% of embryogenesis. Note the prominent commissural complexes anterior (PCC, protocerebral) and posterior (TCC, tritocerebral; MaC, mandibular; Mx:ac,pc, maxillary) to the stomodeum (stom, white dashed outline). Longitudinal fiber tracts link the circumesophageal connectives (coc) with the ventral nerve cord (VNC). Vertical dashed white line indicates brain midline. White dashed rectangle indicates the region containing the somata and initial processes of the VG cluster of cells including the TCG neuron. Arrow points to anterior (A) for all panels. b Reconstruction from whole mount of interneurons in the right DC after repeated extracellular injections with Lucifer Yellow (LY) dye and subsequent anti-LY immunolabeling at 45% of embryogenesis. As in panel a, dashed rectangle represents the region of interest containing neurons of the VG cluster. The axon of the TCG (open black arrow) can be identified from its characteristic contralateral projection via the minor branch of the TCC to the subesophageal ganglion (SOG) where its growth cone (open arrowhead) can be seen extending into the labial neuromere (La). Other axons (black arrowhead) from unidentified neurons of the DC descend ipsilaterally to the SOG via the coc. The commissural system in each neuromere is shaded gray. c Camera lucida drawings from whole mount show the TCG neuron at successive stages of embryogenesis following NiCl2 backfilling from the VNC into the brain. Note the characteristic branch (open black arrowhead, c.f. Fig. 1c for adult) extending laterally and dorsally into the mechanosensory neuropil of the DC. Other abbreviations; fg frontal ganglion. Scale bar represents 100 μm in panel a; 75 μm in panel b; 65 μm in panel c
Discussion
In this study, we document the ontogeny and embryonic development of the TCG neuron, a member of the so-called ventral giant (VG) cluster of cells in the brain of the grasshopper Schistocerca gregaria (Fig. 1; Williams 1972, 1975; Bacon and Tyrer 1978). Labeling against the protein of the segment-polarity gene engrailed places the VG cluster within the deutocerebral neuromere of the embryonic grasshopper brain (Fig. 3), confirming earlier anatomical assumptions from the adult (Williams 1972, 1975; Bacon and Tyrer 1978). We then employ BrdU incorporation and cyclin labeling to visualize proliferative neuroblasts in the early embryonic brain (Fig. 4). Each such neuroblast acquires an intrinsic capacity for neuronal proliferation and generates a specific lineage of progeny via molecular mechanisms which are now largely understood and which are at least partially conserved across species (Broadus and Doe, 1995; Boyan and Reichert, 2011; Reichert, 2011). Since each neuroblast occupies a stereotypic location in the neuroepithelium (Bate 1976; Doe and Goodman 1985; Shepherd and Bate 1990; Ito and Hotta 1992; Zacharias et al. 1993; Urbach and Technau 2003), and the lineages generated maintain their topological positions (Truman et al. 2004; Ito and Awasaki 2008; Shepherd et al. 2016), it is possible to profile such cell clusters temporally, biochemically, and physiologically (Goodman et al., 1979, 1980; Boyan and Liu 2016). When we refer our data to an established map of neuroblasts in the grasshopper brain (Zacharias et al. 1993), we can identify two mitotically active deutocerebral neuroblasts, NB1 and NB6, in a location which suggests they could potentially generate the VG cluster (Fig. 4). Lineage reconstructions allow the VG cluster to then be associated with one of these stem cells, NB1, of the deutocerebrum (Fig. 5).
Lineage of the tritocerebral commissure giant
A neuroblast such as NB1, its progeny, and the tracts they generate can be considered to represent a module or clonal unit, consistent with the mechanism building the association centers of the brain (Ito et al. 1997; Lee and Luo 2001; Ito and Awasaki 2008). As in the ventral nerve cord, the progeny of a given lineage are generated according to a temporal order and maintain their position within the cluster according to birth date so that the lineage acquires a temporal topology (Truman et al. 2004; Williams et al. 2005; Shepherd et al. 2016). At around mid-embryogenesis in the grasshopper, NB1 has generated a lineage of some 20 neurons among which a small group of larger neurons is found at its tip, representing older cells of the lineage (Figs. 2b and 5). The stem cells of the grasshopper brain begin to exit the cell cycle at 70% of embryogenesis and shortly thereafter all undergo programmed cell death (Boyan and Liu 2014), similar to the pattern of events occurring in the ventral nerve cord (Shepherd and Bate 1990). The lineage of NB1 is therefore unlikely to grow significantly beyond the timeframe of our present study and so remains small compared, for example, with the W, X, Y, Z lineages of the central complex of the grasshopper (Boyan et al. 2010) and Drosophila (Izergina et al. 2009) brain where intermediate amplifying progenitors contribute significant numbers of additional progeny to the lineage (Bello et al. 2008; Doe 2008; Boyan et al. 2010; Boyan and Reichert 2011). The fewer cell numbers therefore facilitate lineage analysis and cell identification compared to other regions of the brain.
Previous studies (Williams 1972, 1975; Bacon and Tyrer 1978) and our data here (Fig. 2a) show that the cells belonging to the adult VG cluster have stereotypic cortical soma locations and neuropilar projections into tracts of the deutocerebral striatum. These same morphological characteristics are seen in the cells at the tip of the lineage of NB1 (Fig. 2b), suggesting that they are the embryonic versions of the adult VG neurons. Intracellular dye injection coupled with neuron-specific HRP-labeling in embryonic brain slices (Fig. 6) reveals neurons associated with NB1 having soma locations and axon projections consistent with their being the VG1 (TCG) and VG3 cells. Further, their organization within the lineage (Figs. 2, 4, and 5) leads us to speculate that they might derive from successive cell cycles of the same neuroblast.
Development of the tritocerebral commissure giant
The adult VG cells have been shown to be multimodal interneurons, with the TCG having regulatory, and command-like roles in flight behavior (Bacon and Tyrer 1978; Bacon and Möhl 1979, 1983; Bicker and Pearson 1983; Möhl and Bacon 1983). Although their somata are located close together in the so-called crotch region of the brain (Fig. 1a), the axons of VG1 (TCG) and VG2 project via the ipsilateral circumesophageal connective towards the ventral nerve cord, while that of VG3 projects into the contralateral, circumesophageal connective (Figs. 1 and 2). The TCG establishes its axon prior to 40% of embryogenesis (Müller 1995), and dye injections show that this axon traverses the tritocerebral commissure (TCC) shortly thereafter (Fig. 7). The TCC itself is pioneered by Fasciclin I-positive neurons deriving from neuroblast 12 at the posterior border of the deutocerebrum with the tritocerebrum (Boyan et al. 2003). The TCG does, however, pioneer the minor branch of the TCC via which it, and the tritocerebral dwarf (TCD), projects into the contralateral circumesophageal connective (Figs. 1c and 7b; Bacon and Tyrer 1978; Tyrer et al. 1988; Kutsch and Hemmer 1994). The prominent lateral projections of the TCG into mechanosensory neuropil of the protocerebrum develop early so that the adult morphology of the TCG is already recognizable by mid-embryogenesis (Fig. 7c). This developmental plan matches that reported for other identified brain neurons such as the DCMD (Bentley and Toroian-Raymond, 1981) and Term-1 (Meier et al. 1993; Ludwig et al. 1999, 2001) suggesting that brain circuitry for behaviors such as flight motor activity (Stevenson and Kutsch 1988) and visual collision avoidance (Simmons et al. 2013) is already being assembled during embryogenesis in order to be functional at hatching.
From a cortical perspective, the VG neurons only acquire their very large (giant) soma size with respect to other cells of the cluster after 60% of embryogenesis (Figs. 2 and 7). In lineages of the central complex, early-born neurons are larger than subsequent progeny (Boyan et al. 2008) and this developmental pattern is also true for lineages of the ventral nerve cord (Goodman et al. 1980) where an increase in soma size during development has been associated with a significant upregulation of transmitter or peptide synthesis (Goodman et al. 1979; O'Shea and Adams 1986). Morphological differences among the lineage-related VG cells of the brain such as their varying axon projections into the circumesophageal connectives (Fig. 1b) also have a precedent among successive progeny of neuroblast 7–4 (Bastiani et al. 1984) and the DUM neuroblast (Goodman et al. 1980) in the ventral nerve cord. Physiologically too, differences in spiking vs. non-spiking properties have been documented between early- and later-born cells of the same lineage in the grasshopper (Goodman et al. 1980) and provide a mechanism by which homologous progeny of a common neuroblast can regulate different adult behaviors such as flight, jumping, and walking (Goodman et al. 1979, 1980; Robertson et al. 1982; Thomas et al. 1984; Pearson et al. 1985; Boyan 1992). In this vein, our present analysis offers the possibility of identifying further homologous progeny from deutocerebral NB1, reconstructing their circuitry, and therefore understanding a brain connectome from a developmental perspective.
Evolutionary aspects
To our knowledge, the TCG represents the first anatomically, and functionally, identified insect brain neuron for which an ontogeny can be proposed and so provides an opportunity to further our understanding of evolutionary processes at the single cell level. From a comparative viewpoint, equivalent neuroblasts (e.g., 7–4) and interspecific homologs with recognizable morphologies (such as the equivalent G neurons) have been identified in the VNC of grasshopper, Drosophila and Manduca (Thomas et al. 1984), but their comparative physiological properties and roles in behavior have to date only been elucidated among the serial homologs of NB 7–4 in the grasshopper VNC (Pearson et al. 1985). Neuroblast maps of the embryonic brain now exist for several insect species (Fig. 8a; Urbach and Technau 2003) so that it is possible to propose a putative homologous neuroblast in the DC of each species from which the equivalent TCG might originate. Evidence that conserved developmental programs exist comes from a morphological and physiological study which identified putative TCG homologs in the adult brain of several insects (Fig. 8b; Bacon 1980), all of which receive inputs from the equivalent head sensory organs (head hairs, antennae, compound eyes). Their respective physiological responses, however, reveal differences in the way synaptic input from these various sensory organs is integrated. Ontogenetic, physiological, and morphological analyses of equivalent TCG neurons across species would represent the first time that identified brain neurons could be shown not only to be evolutionarily conserved but reveal how specific adaptations in brain circuitry contribute to adaptive behavior.
Neuroblast maps and homologous neurons in orthopteroid insect species. a Schematics (not to scale) show distributions of neuroblasts for the PC (pink), DC (blue), and TC (green) neuromeres of the embryonic brain in an acridid, a blatted, and a phasmid. Stereotypic locations allow a putative homologous NB1 (shaded black) stem cell for the TCG to be identified in the DC of each species. For simplicity, not all NBs of the TC are depicted. b Reconstructions from whole mount of homologous TCG neurons in the brain of an adult acridid, gryllid, and mantid following intracellular dye injection. Note consistent soma location, dendritic organization, and descending axon via the TCC to the SOG. The minor branch of the TCC is not obvious in all species. Panel a modified from Urbach and Technau (2003), panel b modified from Bacon (1980). Scale bars represent 400 μm
References
Bate CM (1976) Embryogenesis of an insect nervous system. I. A map of the thoracic and abdominal neuroblasts in Locusta migratoria. J Embryol exp Morph 35:107–123
Bate CM, Grunewald EB (1981) Embryogenesis of an insect nervous system. II. A second class of precursor cells and the origin of the intersegmental connectives. J Embryol Exp Morph 61:317–330
Bacon JP (1980) A homologous interneurone in a locust, a cricket and a mantid. Verh dt. zool Ges 1980:163
Bacon JP, Altman JS (1977) A silver intensification method for cobalt-filled neurons in wholemount preparations. Brain Res 138:359–363
Bacon JP, Möhl B (1979) Activity of an identified wind interneurone in a flying locust. Nature 278:638–640
Bacon JP, Möhl B (1983) The tritocerebral commissure giant (TCG) windsensitive interneurone in the locust. I. Its activity in straight flight. J Comp Physiol A 150:439–452
Bacon JP, Tyrer M (1978) The tritocerebral commissure giant (TCG): a bimodal interneurone in the locust, Schistocerca gregaria. J Comp Physiol A 126:317–325
Bastiani MJ, Pearson KG, Goodman CS (1984) From embryonic fascicles to adult tracts: organization of neuropile from a developmental perspective. J Exp Biol 112:45–64
Bentley D, Toroian-Raymond A (1981) Embryonic and postembryonic morphogenesis of a grasshopper interneuron. J Comp Neurol 201:501–518
Bentley D, Keshishian H, Shankland M, Toroian-Raymond A (1979) Quantitative staging of embryonic development of the grasshopper, Schistocerca nitens. J Embryol Exp Morph 54:47–74
Bicker G, Pearson KG (1983) Initiation of flight by an identified wind sensitive neurone (TCG) in the locust. J Exp Biol 104:289–293
Bello BC, Izergina N, Caussinus E, Reichert H (2008) Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev 3:5
Boyan GS (1992) Common synaptic drive to segmentally homologous interneurons in the locust. J Comp Neurol 321:544–554
Boyan GS, Ball EE (1993) The grasshopper, Drosophila, and neuronal homology. Prog Neurobiol 41:657–682
Boyan GS, Hirth F, Reichert H (2003) Commissure formation in the embryonic insect brain. Arthr Struct Devel 32:61–77
Boyan GS, Liu Y, Loser M (2012). A cellular network of dye-coupled glia associated with the embryonic central complex in the grasshopper Schistocerca gregaria. Dev Genes Evol 222:125–138
Boyan G, Liu Y (2014) Timelines in the insect brain: fates of identified neural stem cells generating the central complex in the grasshopper Schistocerca gregaria. Dev Genes Evol 224:37–51
Boyan GS, Liu Y (2016) Development of the neurochemical architecture of the central complex. Front Behav Neurosci 10:167
Boyan GS, Reichert H (2011) Mechanisms for complexity in the brain: generating the insect central complex. Trends Neurosci 34:247–257
Boyan GS, Williams JLD (1995) Lineage analysis as an analytical tool in the insect central nervous system: bringing order to interneurons. In: Breidbach O, Kutsch W (eds) The nervous Systems of Invertebrates: an evolutionary and comparative approach. Birkhäuser Verlag, Basel, pp 273–301
Boyan G, Williams L (2002) A single cell analysis of engrailed expression in the early embryonic brain of the grasshopper Schistocerca gregaria: ontogeny and identity of the secondary headspot cells. Arthr Struct Devel 30:207–218
Boyan G, Williams L, Meier T (1993) Organization of the commissural fibers in the adult and early embryonic brain of the locust. J Comp Neurol 332:358–377
Boyan GS, Therianos S, Williams JLD, Reichert H (1995a) Axogenesis in the embryonic brain of the grasshopper Schistocerca gregaria: an identified cell analysis of early brain development. Development 121:75–86
Boyan GS, Williams JLD, Reichert H (1995b) Morphogenetic reorganization of the brain during embryogenesis in the grasshopper. J Comp Neurol 361:429–440
Boyan GS, Williams JLD, Herbert Z (2008) An ontogenetic anaysis of locustatachykinin-like expression in the central complex of the grasshopper Schistocerca gregaria. Arthr Struct Dev 37:480–491
Boyan GS, Williams L, Legl A, Herbert Z (2010) Proliferative cell types in embryonic lineages of the central complex of the grasshopper Schistocerca gregaria. Cell Tissue Res 341:259–277
Bravo R, Celis JE (1980) A search for differential polypeptide synthesis throughout the cell cycle of Hela cells. J Cell Biol 84:795–802
Bravo R, Macdonald-Bravo H (1987) Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol 105:1549–1554
Broadus J, Doe CQ (1995) Evolution of neuroblast identity: seven-up and prospero expression reveal homologous and divergent neuroblast fates in Drosophila and Schistocerca. Development 121:3989–3996
Burrows M (1996) The neurobiology of an insect brain. Oxford University Press, Oxford
Celis JE, Madsen P, Celis A, Nielsen HV, Gesser B (1987) Cyclin (PCNA, auxiliary protein of DNA polymerase delta) is a central component of the pathway(s) leading to DNA replication and cell division. FEBS Lett 220:1–7
Doe CQ (2008) Neural stem cells: balancing self-renewal with differentiation. Development 135:1575–1587
Doe CQ, Goodman CS (1985) Early events in insect neurogenesis. I. Development and segmental differences in the pattern of neuronal precursor cells. Dev Biol 111:206–219
Ehrhardt E, Kleele T, Boyan G (2015) A method for immunolabeling neurons in intact cuticularized insect appendages. Dev Genes Evol 225:187–194
Goodman CS, Spitzer NC (1979) Embryonic development of identified neurones: differentiation from neuroblast to neurone. Nature 280:208–214
Goodman CS, O'Shea M, McCaman R, Spitzer NC (1979) Embryonic development of identified neurons: temporal pattern of morphological and biochemical differentiation. Science 204:1219–1222
Goodman CS, Pearson KG, Spitzer NC (1980) Electrical excitability: a spectrum of properties in the progeny of a single embryonic neuroblast. Proc Natl Acad Sci U S A 77:1676–1680
Griss C, Rowell CHF (1986) Three descending interneurons reporting deviation from course in the locust I Anatomy. J Comp Physiol A 158:765–774
Hedwig B (2000) Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol 83:712–722
Hedwig B, Heinrich R (1997) Identified descending brain neurons control different stridulatory motor patterns in an acridid grasshopper. J Comp Physiol A 180:285–294
Hensler K (1990) Neural control of optomotor head rolling in locusts. Naturwissenschaften 77:35–37
Hensler K, Rowell CHF (1990) Control of optomotor responses by descending deviation detectors in intact flying locusts. J Exp Biol 149:191–205
Hall PA, Levison DA, Woods AL, CC-W Y, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R, Waseem NH, Lane DP (1990) Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol 162:285–294
Huber F, Markl H (2012) Neuroethology and behavioral physiology: roots and growing points. Springer Science & Business Media, Berlin Heidelberg New York
Ito K, Awasaki T (2008) Clonal unit architecture of the adult fly brain. In: Technau GM (ed) Brain development in Drosophila melanogaster. Springer, New York, pp 137–158
Ito K, Hotta Y (1992) Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol 149:134–148
Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D (1997) The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development 124:761–771
Izergina N, Balmer J, Bello B, Reichert H (2009) Postembryonic development of transit amplifying neuroblast lineages in the Drosophila brain. Neural Dev 4:44
Kien J, Williams M (1983) Morphology of neurons in locust brain and suboesophageal ganglion involved in initiation and maintenance of walking. Proc R Soc Lond (Biol) 219:175–192
Kononenko NL, Pflüger HJ (2007) Dendritic projections of different types of octopaminergic unpaired median neurons in the locust metathoracic ganglion. Cell Tissue Res 330:179–195
Kutsch W, Hemmer W (1994) Ontogenetic studies of flight initiation in Locusta: wind response of an identified interneuron (TCG). J Insect Physiol 40:97–106
Lee T, Luo L (2001) Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24:251–254
Ludwig P, Williams JLD, Lodde E, Reichert H, Boyan GS (1999) Neurogenesis in the median domain of the embryonic brain of the grasshopper Schistocerca gregaria. J Comp Neurol 414:379–390
Ludwig P, Williams JLD, Nässel DR, Reichert H, Boyan GS (2001) Primary commissure pioneer neurons in the brain of the grasshopper Schistocerca gregaria: development, ultrastructure, and neuropeptide expression. J Comp Neurol 430:118–130
Meier T, Therianos S, Zacharias D, Reichert H (1993) Developmental expression of the TERM-1 glycoprotein on growth cones and terminal arbors of individual identified neurons in the grasshopper. J Neurosci 13:1498–1510
Möhl B, Bacon JP (1983) The tritocerebral commissure giant (TCG) wind-sensitive interneurone in the locust. II. Directional sensitivity and role in flight stabilisation. J Comp Physiol A 150:452–465
Müller T (1995) Ontogenetic studies of the TCG-neuron in locusts. Diplomarbeit, Fakultät für Biologie, Universität Konstanz
Ng L, Prelich G, Anderson CW, Stillman B, Fisher PA (1990) Drosophila proliferating cell nuclear antigen. J Biol Chem 266:11948–11954
O'Shea M, Adams M (1986) Proctolin: from “gutfactor” to model neuropeptide. Adv Insect Physiol 19:1–28
O'Shea M, Williams JLD (1974) Anatomy and output connections of the lobular giant movement detector neuron (LGMD) of the locust. J Comp Physiol A 91:257–266
O'Shea M, Rowell CHF, Williams JLD (1974) The anatomy of a locust visual interneurone; the descending contralateral movement detector. J Exp Biol 60:1–12
Patel NH, Kornberg TB, Goodman CS (1989a) Expression of engrailed during segmentation in grasshopper and crayfish. Development 107:201–212
Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS (1989b) Expression of engrailed proteins in arthropods, annelids, and chordates. Cell 58:955–968
Pearson KG, Boyan GS, Bastiani MJ, Goodman CS (1985) Heterogeneous properties of segmentally homologous interneurons in the ventral nerve cord of locusts. J Comp Neurol 233:133–145
Rind FC, Bramwell DI (1996) Neural network based on the input organization of an identified neuron signaling impending collision. J Neurophysiol 75:967–985
Rind FC, Santer RD, Wright GA (2008) Arousal facilitates collision avoidance mediated by a looming sensitive visual neuron in a flying locust. J Neurophysiol 100:670–680
Reichert H (2011) Drosophila neural stem cells: cell cycle control of self-renewal, differentiation, and termination in brain development. In: Kubiak JZ (ed) Cell cycle in development, results and problems in cell differentiation. Springer, Berlin Heidelberg, pp 529–546
Robertson RM, Pearson KG, Reichert H (1982) Flight interneurons in the locust and the origin of insect wings. Science 227:177–179
Rowell CHF (1971) The orthopteran descending movement detector (DMD) neurones: a characterisation and review. Z Vergl Physiol 73:167–194
Santer RD, Rind FC, Stafford R, Simmons PJ (2006) Role of an identified looming-sensitive neuron in triggering a flying locustʼs escape. J Neurophysiol 95:3391–3400
Shepherd D, Bate CM (1990) Spatial and temporal patterns of neurogenesis in the embryo of the locust Schistocerca gregaria. Development 108:83–96
Shepherd D, Harris R, Williams DW, Truman JW (2016) Postembryonic lineages of the Drosophila ventral nervous system: neuroglian expression reveals the adult hemilineage associated fiber tracts in the adult thoracic neuromeres. J Comp Neurol 524:2677–2695
Shreeram S, Blow JJ (2003) The role of the replication licensing system in cell proliferation and cancer. Prog Cell Cycle Res 5:287–293
Simmons P (1980) A locust wind and ocellar brain neurone. J Exp Biol 85:281–294
Simmons PJ, Rind FC (1992) Orthopteran DCMD neuron: a reevaluation of responses to moving objects. II. Critical cues for detecting approaching objects. J Neurophysiol 68:1667–1682
Simmons PJ, Rind FC (1997) Responses to object approach by a wide field visual neurone, the LGMD2 of the locust: characterization and image cues. J Comp Physiol A 180:203–214
Simmons PJ, Sztarker J, Rind FC (2013) Looming detection by identified visual interneurons during larval development of the locust Locusta migratoria. J Exp Biol 216:2266–2275
Stevenson PA, Kutsch W (1988) Demonstration of functional connectivity of the flight motor system in all stages of the locust. J Comp Physiol A 162:247–259
Thomas JB, Bastiani MJ, Bate M, Goodman CS (1984) From grasshopper to Drosophila: a common plan for neuronal development. Nature 310:203–207
Truman JW, Schuppe H, Shepherd D, Williams DW (2004) Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Development 131:5167–5184
Tyrer NM, Pozza MF, Humbel U, Peters BH, Bacon JP (1988) The tritocerebral commissure 'dwarf' (TCD): a major GABA-immunoreactive descending interneuron in the locust. J Comp Physiol A 164:141–150
Urbach R, Technau GM (2003) Early steps in building the insect brain: neuroblast formation and segmental patterning in the developing brain of different insect species. Arthr Struct Devel 32:103–123
Williams JLD (1972) Some observations on the neuronal organisation of the supra-oesophageal ganglion in Schistocerca gregaria Forskål with particular reference to the central complex. PhD Thesis, University of Wales
Williams JLD (1975) Anatomical studies of the insect central nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J Zool Lond 176:67–86
Williams JLD, Guentner M, Boyan GS (2005) Building the central complex of the grasshopper Schistocerca gregaria: temporal topology organizes the neuroarchitecture of the w, x, y, z tracts. Arthr Struct Devel 3:97–110
Yamaguchi M, Date T, Matsukage A (1991) Distribution of PCNA in Drosophila embryo during nuclear division cycles. J Cell Sci 100:729–733
Yamaguchi M, Nishimoto Y, Hirose F, Matsukage A (1995) Distribution of PCNA during postblastoderm cell division cycles in the Drosophila melanogaster embryo: effect of a string- mutation. Cell Struc Funct 20:47–57
Zacharias D, Williams JLD, Meier T, Reichert H (1993) Neurogenesis in the insect brain: cellular identification and molecular characterization of brain neuroblasts in the grasshopper embryo. Development 118:941–955
Zudaire E, Simpson SJ, Illa I, Montuenga LM (2004) Dietary influences over proliferating cell nuclear antigen expression in the locust midgut. J Exp Biol 207:2255–2265
Acknowledgments
George Boyan and Leslie Williams thank Dr. E. Ball for the gift of the engrailed antibody, Dr. E. Ehrhardt for assistance with the confocal microscopy, and Karin Fischer for general technical assistance. George Boyan and Leslie Williams received support from the Deutsche Forschungsgemeinschaft (BO 1434/3-5) and the Graduate School of Systemic Neuroscience, LMU. Tobias Müller acknowledges the personal support of Prof. Dr. W. Kutsch, Universität Konstanz, and received financial support from the Deutsche Akademische Austauschdienst (DAAD) and from ARC grant 313-ARC-VII 93/50 to Jonathan Bacon.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in accordance with the guidelines for animal welfare as laid down by the Deutsche Forschungsgemeinschaft.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by Claude Desplan
Rights and permissions
About this article
Cite this article
Boyan, G.S., Williams, L., Müller, T. et al. Ontogeny and development of the tritocerebral commissure giant (TCG): an identified neuron in the brain of the grasshopper Schistocerca gregaria. Dev Genes Evol 228, 149–162 (2018). https://doi.org/10.1007/s00427-018-0612-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-018-0612-0