Abstract
The signaling factor Wingless regulates multiple steps during the postembryonic development of the Drosophila eye. To obtain insight into the molecular regulation of embryonic eye development in primitive insects, we studied the expression of wg and genes projected to interact with wg in the grasshopper Schistocerca americana. We find that the dynamic and complex expression of wg in the early grasshopper procephalon results in three paired expression domains with relevance to eye primordium development. By comparison with Drosophila, these domains are compatible with a conserved function of wg during anteroposterior and dorsoventral axis formation by repression of retinal differentiation and stimulation of tissue proliferation. This is further supported by the expression of grasshopper orthologs of the retina determination genes sine oculis and eyes absent, and by inhibition of retina differentiation in grasshopper eye primordia cultured with LiCl. Surprisingly, the expression of wg and the grasshopper orthologs of pannier, fringe, Delta, and Iroquois complex is inconsistent with induction of midline centered Notch signaling activity, which is essential for Drosophila retina development. Similarly substantial evolutionary divergence is found concerning the control of retina versus head epidermis specification. The transcription factor Extradenticle (Exd), which cooperates with wg in specifying the Drosophila head epidermis, is not detected outside the labral and antennal primordia in the embryonic grasshopper head. Our results, which provide the first insight into the molecular control of eye primordium formation in primitive insects, suggest substantial modification of this process during the evolution of the Drosophila mode of postembryonic eye development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drosophila wingless (wg) is a founding member of the highly conserved wnt gene family of small signaling factors characterized by a string of conserved cysteine residues (Rijsewijk et al. 1987). Multiple wnt gene family members have been discovered in Drosophila and vertebrates (for review, see Cadigan and Nusse 1997). Similarly conserved is the downstream cascade of signal transduction protein interactions (for review, see Cadigan 2002). In the canonical Wnt signaling pathway, the activity state of a cell is determined by the concentration of cytosolic Armadillo/β-catenin protein. In Wnt signaling silent cells, Armadillo/β-catenin is constitutively degraded upon phosphorylation by Glycogen synthase kinase-3β (GSK-3β). Wnt ligand binding to the Frizzled and Arrow/LRP co-receptor complex leads to repression of GSK-3β-mediated phosophorylation allowing Armadillo/β-catenin to translocate into the nucleus and join the formation of a transcriptional activation complex which includes DNA-binding proteins of the dTCF/LEF family (van de Wetering et al. 1997). The canonical Wnt signaling pathway seems to operate in the majority of wnt-dependent patterning events. Alternative Wnt signaling-related pathways, which participate in planar polarity patterning (Fanto and McNeill 2004) and vertebrate germ layer specification (Saneyoshi et al. 2002; Slusarski et al. 1997), are activated by wnt factors different to the wnt1 orthology group to which wg belongs.
The wg gene was originally identified due to its segment polarity gene function in the Drosophila embryo, which is highly conserved in insects (Nagy and Carroll 1994; Nüsslein-Volhard and Wieschaus 1980; Oppenheimer et al. 1999). Subsequent studies revealed involvement in a multitude of patterning processes including development of the visual system (Kaphingst and Kunes 1994; Ma and Moses 1995; Treisman and Rubin 1995). In combination with other long range signaling factors such as Hedgehog (Hh) and the TGF-β homolog Decapentaplegic (Dpp) Wg provides global patterning cues which control many steps of Drosophila retina development (Lee and Treisman 2002). Recent studies have identified multiple patterning functions of wg during early and late Drosophila retina development (Baonza and Freeman 2002; Cadigan et al. 2002; Cavodeassi et al. 1999; Lee and Treisman 2001; Lin et al. 2004; Maurel-Zaffran and Treisman 2000; Pichaud and Casares 2000; Singh et al. 2002; Tomlinson 2003; Wernet et al. 2003).
The Drosophila visual system forms by partitioning of a pair of homogenous embryonic anlagen into that of the larval eye, the adult retina initially segregating with the eye-antennal imaginal disc, and the optic lobe (Younossi-Hartenstein et al. 1993; Green et al. 1993). The latter encompasses three separate ganglia: lamina, medulla, forming the outer optic lobe, and lobula, forming the inner optic lobe (Meinertzhagen and Hanson 1993). Earliest wg expression in the visual system is seen in the outer optic lobe anlagen. A pair of single cells marking the dorsal and ventral margin of the future lamina and medulla neuropils start expression of wg soon after hatching of the first instar larva (Kaphingst and Kunes 1994). The number of wg-expressing cells increases to form small polar outer optic lobe expression domains at the beginning of the third larval instar. Size reduction and delayed cell fate marker expression in a wg mutant background indicate involvement of Wg signaling in growth activation and cell fate specification in the outer optic lobe anlage (Kaphingst and Kunes 1994). In addition, wg has been implicated in dorsoventral axis polarity formation in the outer optic lobe anlage based on wg-regulated expression of dpp, Distal-less (Dll) and optomotor-blind (omb; Kaphingst and Kunes 1994).
The regulation of wg expression is more complex in the eye-antennal imaginal disc, which, in addition to the adult eye, generates antenna, ocelli and large parts of the dorsal and ventral head cuticle during postembryonic development of the larva (Haynie and Bryant 1986; Fig. 1). Wg expression has been reported for freshly hatched first instar larvae when the eye-antennal imaginal disc represents a flat compact sac of undifferentiated tissue (Cho et al. 2000). Based on immunohistochemical detection, Wg expression levels are uniform in first and second instar eye-antennal imaginal discs (Pichaud and Casares 2000; Royet and Finkelstein 1996, 1997). Reporter gene expression analysis however indicates early restriction of wg-expressing cells to a region corresponding to the dorsal hemisphere of the future adult head (Cho et al. 2000). Moreover, these cells seem to be part of the peripodial membrane layer of the sac-like disc as opposed to the disc-proper layer from which the actual retina develops (Cho et al. 2000; Gibson and Schubiger 2000). Throughout most of the second larval instar, wg remains predominantly expressed in the dorsal domain of the eye antennal disc. Its ventral expression border runs very precisely along the dorsoventral midline of the disc (Cho et al. 2000; Treisman and Rubin 1995). At the end of the second instar, wg starts to clear from the posterior pole of the disc. A new expression domain is detected in the ventral anterior margin after the eye-antennal disc has been partitioned into morphologically distinct antennal and eye field anlagen (Cho et al. 2000; Ma and Moses 1995; Treisman and Rubin 1995). wg continues to be expressed in the dorsal and ventral margins anterior to the presumptive eye field throughout the third larval instar (Baker 1988b). In the dorsal margin, wg expression splits into two patches, which correspond to the anlagen of the lateral orbital region and the ptilinium of the head cuticle (Royet and Finkelstein 1996). A newly emerging expression domain outlining the posterior margin of the late third instar eye disc is thought to eventually form the narrow rim of wg-expressing cells surrounding the terminally differentiating retina in the pupa (Cadigan et al. 2002; Tomlinson 2003).
Schematic overview of wingless expression and function in the Drosophila retina. In this and all other figures, dorsal is up and anterior to the left unless indicated otherwise. a Induction of Notch signaling organizer by dorsal compartment expression of wg in the second instar eye-antennal imaginal disc. b Spatial regulation of the origin point of retina differentiation at the posterior margin of the eye disc by clearance of wg from the posterior region coincident with the emergence of a ventral anterior expression domain in the early third instar. c Constant expression of wg in the anterior margin of the eye imaginal disc in the late third laval instar associated with head cuticle versus retina cell fate determination and negative regulation of furrow progression. d Patterning of the retina margin by wg expressed in a ring around the pupal eye. mf Morphogenetic furrow
The dynamic regulation of wg expression in the Drosophila eye-antennal imaginal disc is the consequence of regulatory multitasking during progressive cell fate specification in the developing disc.
(1) The early dorsal expression of wg is an essential component of a gene network, which divides the eye primordium into dorsal and ventral compartments. The border of these compartments runs along the dorsoventral midline of the disc. It was first described as the equator of the adult retina, the separation line of dorsal and ventral fields of ommatidia with mirror image orientation (Dietrich 1909). The equator is also an important patterning coordinate in the developing Drosophila eye. In the early eye disc, wg activates the expression of the Iroquois complex (Iro-C) genes mirror (mirr), caupolican (caup) and araucan (ara), which code for highly related homeodomain transcription factors (Gomez-Skarmeta et al. 1996; McNeill et al. 1997). The expression of Iro-C selector genes in the dorsal compartment restricts expression of the Notch (N) signaling modulator fringe (fng) to the ventral compartment (Cho and Choi 1998; Dominguez and de Celis 1998; McNeill et al. 1997; Yang et al. 1999). Analogous to the specific activation of N signaling in the Drosophila wing margin (Panin et al. 1997), the resulting fng expression border triggers the build-up of a strong N signaling center along the eye disc midline, which is essential for growth and differentiation in the retina primordium (Cho and Choi 1998; Dominguez and de Celis 1998; Papayannopoulos et al. 1998).
(2) The subsequent withdrawal of expression to the anterior part of the eye disc turns wg into an instructive cue of anterioposterior and dorsoventral polarity (Lee and Treisman 2001). Signaling from both the dorsal and ventral pole in the anterior margin, Wg restricts the initiation of retina differentiation to the posterior margin midline by blocking photoreceptor differentiation and repressing Dpp activity in the lateral eye disc margins (Ma and Moses 1995; Reifegerste and Moses 1999; Treisman and Rubin 1995).
(3) During the dynamic differentiation of the retina led by the morphogenetic furrow, the persistent dorsal and ventral wg expression domains anterior to the presumptive eye field regulate proper allocation of eye disc tissue to eye and head cuticle primordia (Royet and Finkelstein 1997). This is associated with stimulation of tissue growth (Baonza and Freeman 2002; Bessa et al. 2002; Lee and Treisman 2001), activation of retina-differentiation-inhibiting genes (Pichaud and Casares 2000), and repression of retinal determination and neuronal differentiation genes (Baonza and Freeman 2002; Cadigan et al. 2002).
(4) Once the morphogenetic furrow has traveled the entire prospective eye field, Wg emanating from the periphery into the retina field patterns the retina margin by inducing pigment rim formation (Tomlinson 2003), repressing bristle cell formation (Cadigan et al. 2002; Cadigan and Nusse 1996; Tomlinson 2003) and inducing apoptosis in peripheral ommatidia with aberrant numbers of photoreceptor cells (Lin et al. 2004).
(5) In the dorsal hemisphere of the retina, the combination of pupal Wg signaling and Iro-C selector gene expression induces the specification of the polarized-light-sensitive ommatidia of the dorsal rim area (Tomlinson 2003; Wernet et al. 2003).
The persistent involvement of Wg signaling in the Drosophila eye makes this pathway an ideal entry point to explore the developmental organization of eye development in insects other than Drosophila. Orthologs of wg have been isolated from a variety of distantly related insects and other arthropods (Damen 2002; Duman-Scheel et al. 2002a; Friedrich and Benzer 2000; Hughes and Kaufman 2002; Janssen et al. 2004; Nagy and Carroll 1994; Niwa et al. 2000; Nulsen and Nagy 1999). In flour beetle, grasshopper and a malacostracan crustacean, wg expression domains have been described anterior of the differentiating retina, which are very similar to that in the late Drosophila eye disc (Duman-Scheel et al. 2002a; Friedrich and Benzer 2000). This is remarkable considering the different mode of retina anlagen morphogenesis in more primitive arthropod species (Friedrich 2003). In hemimetabolous insects, such as grasshopper, differentiation of the adult eye initiates during embryonic development (Friedrich and Benzer 2000). In the postgastrulation embryo, neuroblasts delaminate from the lateral procephalon. Massive proliferation of these neuroblasts yields the outer optic lobe anlagen, which initially protrude from the embryonic head thereby forming the conspicuous embryonic eye lobes. The anlagen of the adult eyes are contained in the eye lobe covering ectoderm. Their differentiation is initiated at the posterior margin of the eye lobe ectoderm in the form of a morphogenetic furrow. This embryonic furrow moves, as in the Drosophila eye disc, in direction of the anterior margin of the future eye initiating the cell fate determination and cell differentiation processes associated with the development of ommatidia (Friedrich and Benzer 2000; Dong et al. 2003). The regular array of ommatidia formed in the embryo develops in the compound eye of the first instar nymph, which is maintained into the adult as the posterior region of eye supplemented by postembryonic development of additional anterior ommatidia. In summary, cell fate determination and differentiation are very similar in the grasshopper and Drosophila eye. Less commonality, however, seems to exist between the postembryonic formation of the eye anlage in Drosophila and the embryonic formation of the eye primordium in close association with the developing optic neuropils in grasshopper.
In this study, we present a comprehensive analysis of the regulation of wg expression during visual system development in the grasshopper Schistocerca americana. This work extends a previous analysis which focused on the possible regulation of wg by dpp in the grasshopper eye (Friedrich and Benzer 2000). We find that wg is expressed in a complex and dynamic pattern during grasshopper embryonic eye development. To further understand the role of wg in this system we investigate the expression of grasshopper orthologs of genes which interact with wg during dorsoventral compartment formation, equator patterning, and head cuticle versus retina determination in Drosophila. These data are complemented with an analysis of the effect of stimulating Wg signaling in cultured grasshopper eye primordia with LiCl. Our findings indicate conservation of wg involvement in negative control of retina differentiation, dorsoventral and anteroposterior axis formation, and tissue growth stimulation in the retina primordium, as well as dorsoventral axis formation and tissue growth stimulation in the developing optic lobe. However, significant differences between Drosophila and Schistocerca are revealed regarding the role of dorsoventral patterning genes and N signaling in patterning of the retina primordium.
Materials and methods
Animal culture
Adults and embryos of the American desert locust Schistocerca americana were raised and processed as previously described (Dong et al. 2003). Embryos were staged following morphological criteria established by Bentley et al. (1979).
Cloning and sequence analysis
The degenerate PCR approach was taken to isolate portions of the grasshopper orthologs of the Drosophila genes eyes absent (Sa_eya), sine oculis (Sa_so), Delta (Sa_Dl), pannier (Sa_pnr) as well as of a gene homologous to the members of the Drosophila Iro-C (Sa_iro). The RNAqueous kit (Ambion) was used to isolate total RNA from S. americana embryos ranging from 35% to 45% of development followed by mRNA purification with PolyATract System III (Promega). cDNA template was prepared either from total RNA or mRNA preparation with random decamers or Oligo(dT) using the RETROscript kit (Ambion). Initial PCR amplifications were carried out using nested sets of degenerate primers against conserved gene regions determined by alignment of published homologs from D. melanogaster, Anopheles gambiae and mammalian species. PCR fragments were cloned into the pGEM-T vector system (Promega) and plasmid DNA was purified with the Perfectprep Plasmid Mini kit (Eppendorf) for sequencing with the BigDye Terminator Sequencing kit (Applied BioSystems). Sequence reads were prepared on an Applied Biosystems ABI Prism 3700 sequencer in the Applied Genomics Technology Center of Wayne State University. In the case of Sa_iro, ten individual clones were sequenced all of which were of identical sequence suggesting the presence of a single iro-C gene homolog in grasshopper.
PCR fragments obtained from Sa_eya and Sa_Dl were directly used for whole-mount in situ hybridization probe preparation. By combining a degenerate primer targeting a conserved region outside the initially obtained RT-PCR fragment with a specific upstream primer, a 123-bp longer fragment was isolated for generating the antisense probe of Sa_so. In the case of Sa_pnr and Sa_iro, an additional 3′ sequence region was obtained in 3′ RACE experiments with the First-choice RLM-RACE kit (Ambion). Gene specificity of amplified 3′ RACE products was confirmed by non-radioactive Southern blot hybridization with digoxigenin-labeled DNA probes (DIG DNA Labeling and Detection kit; Roche Biochemicals) before cloning into the pGEM-T vector system.
Sequence analysis and alignment were carried out with MacVector 7.2 (Oxford Molecular Group) and Clustal W (Thompson et al. 1994). Regions spanning unambiguously conserved amino acid sites were used for gene tree estimation with neighbor-joining as implemented in Clustal W (Thompson et al. 1994). PCR primer sequences, amino acid sequence alignments and gene trees are available as Electronic Supplementary Material file. Accession numbers, PCR fragment lengths, corresponding residues and functional domains in the Drosophila homologs are described in Table 1.
Whole-mount in situ hybridization
Gene fragments obtained either from 3′ RACE or nested RT-PCR were used to serve as templates for labeling in vitro transcription (DIG and Fluorescein RNA Labeling Kit, Roche Biochemicals). The digoxygenin-labeled antisense RNA probes were applied to whole-mount in situ hybridization as described (Friedrich and Benzer 2000).
For Sa_eya and Sa_wg double labeling in situ hybridization, we generated digoxigenin-labeled Sa_eya antisense RNA and fluorescein-labeled Sa_wg antisense RNA. After detection of the digoxigenin-labeled Sa_eya probe as described above, antiDIG-AP Fab fragments were removed by incubation in 0.1 M glycine hydrochloride/0.1%Tween 20, pH 2.2, for 10 min, followed by washing in 0.1 M maleic acid buffer for 4×5 min and 1-h preincubation in 1× blocking buffer. The second detection was carried out by incubating for 1 h with anti-fluorescein-AP (Roche) at 1:5,000 dilution. Embryos were then washed with 0.1 M maleic acid buffer for 4×20 min before pursuing detection with Fast Red substrate (Roche). Brightfield images were taken with a SPOT RT CCD digital camera (Diagnostic Instruments) coupled to a Zeiss Axioscope.
Immunohistochemistry
Embryos were dissected and fixed in 4% formaldehyde in PEMS buffer for 20 min followed by dehydration with 95% ethanol and rehydration with 0.1% Triton X PBS buffer. Afterwards, the embryos were preincubated in PBS 0.1% Triton X/0.1% BSA for 30 min. Mouse anti-Drosophila Extradenticle (Exd) monoclonal antibody B11M (Aspland and White 1997), which cross-reacts with Exd protein in a wide range of arthropods (Abzhanov and Kaufman 2000), was used at 1:5 dilution. Alexa488-labeled goat anti-mouse secondary antibody (Molecular Probes) was used at a dilution of 1:500. After staining, the embryos were cleared in 70% glycerol/PBS supplemented with 2.5% DABCO and individually mounted for confocal analysis.
In vitro culturing
Grasshopper embryonic eye lobe cultures were initiated as described previously based on a modified protocol for grasshopper primary tissue cultures (Dong et al. 2003; Myers and Bastiani 1993). Per embryo, one eye lobe was fixed immediately to serve as the 0-h reference. The second eye lobe was incubated for 48 h, which results in the formation of approximately six rows of new ommatidial preclusters (Dong et al. 2003). LiCl (Fisher Scientific) was dissolved in PBS to 1 M stock concentration. In dosage response experiments ranging from 1 to 80 mM final concentration, strong inhibition of retina differentiation was observed at a minimal concentration of 40 mM.
Analysis of retina development
Cytoskeletal morphology and chromatin conformation were visualized by double labeling with AlexaFluor 633-conjugated phalloidin (Molecular Probes), and propidium iodide following published protocols (Friedrich and Benzer 2000; Orsulic and Peifer 1994). Confocal image stacks were collected with a Leica TCS SP2 laser scanning confocal microscope and processed with Leica Confocal Software, ImageJ (http://rsbweb.nih.gov/ij/) and Adobe Photoshop 6.0. To quantify the progress of retina differentiation, the number of ommatidial columns along the eye lobe midline was counted. Quantitative analysis of mitotic and apoptotic cell frequency was performed by comparison of the average numbers of mitotic cells anterior and posterior to the morphogenetic furrow counted within a window size of 246×164 μm. Averages were derived from a total of ten samples prepared in two independent experiments.
Results
Complex regulation of wg expression during grasshopper eye primordium development
The early expression of wg in the grasshopper embryonic head is consistent with global patterning functions. As previously reported (Dearden and Akam 2001), wg is strongly expressed in the head lobes of the early germband, which comprise the paired anlagen of the procephalic neuropils (proto-, deutero-, and tritocerebrum) and associated head epidermis. A pair of large, irregularly shaped procephalic expression domains can be detected prior to morphologically visible segmentation or head compartment specification (Fig. 2a). Derivatives of these primordial procephalic wg expression domains can be tracked into the late second half of embryogenesis, when a part of them merges into the anterior margin of the eye lobe ectoderm (Fig. 2g–j). In the early embryo, their peripheral border looks close to a straight line which runs at an approximately 30° angle in relation to the anteroposterior body axis (Fig. 2a, b). This border is about one to two cell diameters away from the head lobe margin (Fig. 2a) widening to three to four cells at subsequent stages (Fig. 2b, c). At the anterior end of the expression domain, wg-positive cells extend further towards the midline than at the posterior end. This gives the procephalic wg expression domain an almost boomerang-like appearance.
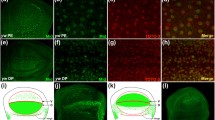
Complex regulation of wingless expression in the developing grasshopper visual system. Ventral view of embryonic head to show the dynamics of procephalic wg expression domains (stars) at 15% (a), 20% (b), 23% (c) and 30% (d) of development. Frontolateral perspective of eye lobe flat preparations at 30% (e), 33% (f), 38% (g), 45% (h), 55% (i) and 65% (j) of development. Outer optic lobe anlagen domains out of focus in f–j. Arrowheads in f indicate the borders of the tissue fold which separates protocerebrum and eye lobe. Arrows in j point to out of focus wg expression in the posterior margin of the late stage eye lobe. In this and all other figures, scale bars correspond to 100 μm unless indicated otherwise. ant Antenna, DOA dorsal optic lobe anlagen domain, DPN dorsal protocerebral neuroectoderm domain, DPE dorsal protocerebral ectoderm domain, ele eye lobe ectoderm, lbr labrum, man mandible, MPN median protocerebral neuroectoderm domain, olo outer optic lobe, pro procephalon, sto stomodeum, VOA ventral optic lobe anlagen domain, VPE ventral protocerebral ectoderm domain, VPN ventral protocerebral neuroectoderm domain
Between stage 15% and 20% of development, the procephalic wg expression domains widen concurrent with head lobe enlargement (Fig. 2b). Once the procephalic tissue sheet begins to organize into a three-dimensional structure, the procephalic wg domains condense into short stripes with small extensions branching off at both ends (Fig. 2c). Each of the procephalic domains eventually separates into three discrete daughter-domains. The smallest derivative, which is given off in a median direction, does not contribute to eye primordium patterning and is not considered further here. The peripheral derivatives are considerably larger. They take polar positions at the lateral edge of the developing protocerebrum, where it borders the emerging compound eye primordia, the eye lobes (Fig. 2d, e).
During morphogenesis of the prognathous grasshopper head capsule, the procephalic head compartments lift up by about 90° with respect to the anteroposterior body axis (Boyan et al. 1995). The anterior regions of the embryonic procephalon thereby shift into the dorsal half of the head, while the posterior regions remain ventral. If the adult body axis coordinates are projected onto the early embryonic primordia, the median eye lobe border, which faces the protocerebrum, corresponds to the anterior margin of the future compound eye. Its prospective dorsal pole points towards the anterior of the embryo, and the prospective ventral pole towards the posterior. In the remaining description of protocerebrum and eye lobe development, we will refer to their coordinates in the adult head.
Thus, upon completion of their separation, the two procephalic wg domains take opposite positions along the dorsoventral axis in front of the developing eye lobe. The larger of the two domains seems to cover the entire dorsal pole, while the smaller domain comes to rest close to the ventral-most pole of the eye field. These domains continue to share several characteristics. They are of roundish outline, consist of small, cuboidal neuroectodermal cells (Fig. 2e), and contribute transiently wg-expressing neuroblasts to the developing protocerebrum (Fig. 2f). Neuroblasts are given off in two directions, into the periphery towards the optic lobe anlagen and in a median direction towards the protocerebrum. We will refer to these expression domains as the dorsal and ventral protocerebral neuroectoderm domains.
At 30% of development, the dorsal-most protocerebral neuroectoderm domain appears to leap into dorsal head margin cells (Fig. 2d). Close examination of flattened tissue preparations confirms a broad band of wg-positive cells at the dorsal head lobe margin which seem close to but separate from the dorsal protocerebral neuroectoderm domain (Fig. 2e). Unlike the cells of the protocerebral neuroectoderm domain, the cells in the head lobe margin appear columnar and of epidermal character. The initial separation of these neuroectodermal and ectodermal wg-expressing cell populations suggests that the latter is not derived from the former but that wg expression is de novo activated in ectodermal cells. This initiation of dorsal ectodermal expression marks a distinct step in the diversification of wg expression domains in the grasshopper embryonic procephalon. Notably, no complementary wg expression is seen in the ventral head lobe ectodermis at this point of development (Fig. 2e).
Coincident with the initiation of wg expression in the dorsal head lobe margin, wg expression is for the first time seen within the eye primordium proper in the form of one to two cell large expression domains at the polar edges of the outer optic lobe anlage (Fig. 2e). These previously described domains (Friedrich and Benzer 2000) will be referred to as dorsal and ventral outer optic lobe domains. While expression of the outer optic lobe domains persists without major change except for cell number increase into late embryogenesis (>65% of development), the ectodermal and neuroectodermal protocerebral domains proceed through further significant changes. The position of the dorsal ectodermal domain gradually shifts from the lateral protocerebrum into a pronounced tissue fold separating protocerebral and eye lobe compartments (Fig. 2f). At the same stage, a small rim of weak wg expression can for the first time be detected in the ventral eye lobe ectoderm immediately adjacent to the protocerebral border (Fig. 2f). The asynchronous activation of these polar wg expression domains in the protocerebral ectoderm stands in sharp contrast to the highly synchronized appearance of the outer optic lobe anlagen domains.
Following the initiation of retina differentiation shortly before 35% of development, the strong dorsal ectodermal wg domain continues to shift towards the eye lobe compartment until it is eventually incorporated in the anterior margin of the eye lobe proper (Fig. 2g–j). In parallel, expression of the ventral protocerebral ectoderm expression domain increases reaching levels equivalent to that in the complementary dorsal domain (Fig. 2g–j). This process is complemented by the apparent fusion of the ectodermal domains with the more median neuroectodermal domains, which acquire ectodermal properties and thin out into sharp expression domains at the anterior eye lobe border (Fig. 2i, j). By 55% of embryonic development, neuroectodermal wg expression aspects have largely disappeared from the developing procephalon (Fig. 2i). During further differentiation of the eye lobes, the polar ectodermal wg domains extend further medially both at the anterior and posterior margin of the eye lobe, highly reminiscent of wg expression surrounding the Drosophila eye during pupation (Cadigan et al. 2002; Fig. 2j).
Compartment formation in the grasshopper embryonic retina based on expression of the Iro-C and pnr orthologs
The emergence of wg expression domains in front of the grasshopper developing retina shows similarities and differences to Drosophila. Unlike Drosophila, the polar expression domains are established with or by the beginning of eye primordium formation in the form of large neuroectodermal expression domains. Yet similar to Drosophila, wg is activated earlier in the dorsal than in the ventral ectoderm. Nonetheless, at no stage is wg expression leaping into the dorsal half of the future eye field as in the Drosophila eye disc (Fig. 2). This indicates possible differences in the molecular genetic control of dorsoventral compartment formation during grasshopper eye development. We therefore studied the expression of two transcription factors, which interact with wg during dorsoventral compartment specification in Drosophila. The GATA family transcription factor pannier (pnr) initiates wg expression in the dorsal compartment of the Drosophila eye disc (Maurel-Zaffran and Treisman 2000; Ramain et al. 1993). Dorsally expressed wg in turn activates the expression of the Iro-C genes (Cavodeassi et al. 1999; Gomez-Skarmeta et al. 1996; McNeill et al. 1997), which specify the dorsal compartment of the Drosophila eye field (Cavodeassi et al. 1999; Heberlein et al. 1998). To investigate whether this genetic network is conserved, we cloned portions of the grasshopper orthologs of Drosophila pnr (Sa_pnr) and the Drosophila Iro-C family (Sa_iro).
Consistent with a conserved role in dorsal patterning, Sa_pnr is exclusively expressed in the dorsal-most cells of the grasshopper germband corresponding to the Drosophila leading edge cells (Fig. 3a). This expression domain is shared with Sa_iro (Fig. 3d) and the grasshopper ortholog of dpp (personal observation) indicating that the positive regulatory relationships between dpp, pnr and Iro-C genes described for Drosophila dorsal body wall patterning are conserved in the grasshopper (Calleja et al. 2000; Heitzler et al. 1996).
Dorsoventral compartment formation in the grasshopper embryonic eye field based on the expression of Sa_wingless, Sa_pannier and Sa_Iroqois complex. a, d, g Dorsal view of embryonic head region. Anterior of embryonic axis is up. b, e, h High magnification of dorsal eye lobe. c, f, i Frontolateral view of eye lobe. a–c Expression of Sa_pnr, d–f expression of Sa_iro, g–i expression of Sa_wg. Arrows indicate position of leading edge cells. ele eye lobe ectoderm, elo eye lobe, man mandible, max maxilla, olo outer optic lobe, sto stomodeum
Different from the evolutionarily derived head patterning situation in the Drosophila embryo, leading edge cell expression of Sa_pnr and Sa_iro extends into the head region of the grasshopper embryo (Fig. 3a, d). Interestingly, Sa_pnr expression is confined to the leading edge cells and does not reach into the dorsal eye lobe (Fig. 3b, c) whereas expression of Sa_iro extends from the leading edge cells into the dorsal margin of the eye lobe ectoderm (Fig. 3e, f). The Sa_iro expression domain occupies approximately the dorsal-most 10% of the eye lobe ectoderm tapering off in a gradient-like manner in the direction of the eye lobe midline (Fig. 3f). In the adjacent dorsal head ectoderm, Sa_iro expression is shifted ventrally compared to that of Sa_pnr. Sa_iro clearly overlaps with the dorsal ectodermal Sa_wg domain (Fig. 3e, h). Sa_wg and Sa_pnr, however, are expressed in clearly non-overlapping patterns (compare Fig. 3b, h). In summary, these observations rule out activation of wg by pnr but are compatible with activation of Sa_iro by wg in the very dorsal margin of the grasshopper eye lobe. The lack of midline patterning-related expression of Sa_iro suggests involvement in dorsal-margin- but not midline-related compartment specification.
Spatial regulation of N signaling in the grasshopper retina field based on expression of the grasshopper orthologs of fng and Dl
The immediate molecular readout of wg-controlled dorsoventral compartment formation in the Drosophila eye disc is the compartment-specific expression of N signaling ligands and modulators. Wg activates dorsal expression of the Iro-C selector genes, which restrains expression of the glycosyltransferase fng to the ventral compartment. N signaling is specifically activated at the fng expression border along the equator. This is associated with increased expression of Dl dorsal of the equator and Serrate (Ser) ventral of the equator (Irvine and Wieschaus 1994; Panin et al. 1997). The Wg-signaling-dependent compartment-specific expression of Dl, fng and Ser is essential for activation of a N signaling center along the midline of the Drosophila eye disc (Cho and Choi 1998; Dominguez and de Celis 1998; Papayannopoulos et al. 1998). Disruption of the Drosophila N organizing center leads to abortion of eye disc development.
The lack of dorsoventral compartment-specific expression of Sa_wg and Sa_iro raised the possibility that the grasshopper eye field develops without activation of a N-signaling-based organizer. This possibility was further tested by studying the expression of the grasshopper orthologs of Dl (Sa_Dl) and fng (Dearden and Akam 2000). Before onset of retina differentiation, low levels of fng can only be detected in the outer optic lobe anlagen but not in the eye lobe ectoderm (Fig. 4a). Eye lobe ectoderm expression is first detected at 33% of development as a discrete spot at the posterior margin in the upper ventral half of the eye field (Fig. 4b). The onset of this expression domain coincides closely in time and space with the initiation of furrow progression in the grasshopper retina (Dong et al. 2003; Friedrich and Benzer 2000). During subsequent stages of development, this fng domain extends in both dorsal and ventral directions (Fig. 4c, d). At 45% of development fng is expressed as a discrete seven- to nine-cell-wide domain extending from the morphogenetic furrow in an anterior direction (Fig. 4e). A sharp drop of expression is seen posterior to the furrow in contrast to gradual attenuation anterior to the furrow. Expression of fng in the morphogenetic furrow is also observed in Drosophila (Cho and Choi 1998), where it correlates with the role of N and Dl in promoting furrow initiation and progression (Baker 2002; Baonza and Freeman 2001; Kumar and Moses 2001). The ventral expression of fng anterior to the Drosophila morphogenetic furrow, however, is not observed in the grasshopper. Thus, a regulatory key component of the network triggering midline activation of N signaling in Drosophila is not conserved in the grasshopper.
Spatial regulation of Notch signaling in the grasshopper retina field based on expression of fringe and Sa_Delta. Frontolateral view of eye lobe at 27% (a), 33% (b), 35% (c) and 37% (d) of development. b Stars indicate expression in the outer optic lobe anlage. Red arrow indicates the localization of furrow initiation as indicated by fng expression. Solid red arrowhead points at the eye lobe midline as indicated by the median dent in the posterior margin of the outer optic lobe anlage. e Lateral view of 45% stage eye lobe labeled for fng expression. Black arrows indicate the morphogenetic furrow. f Lateral view of 45% stage eye lobe for Sa_Dl expression. The broad band of expression (arrowheads) anterior to the morphogenetic furrow is localized in the optic lobe anlage and not in the retina. Black arrows indicate the morphogenetic furrow as in e. ant Antenna, ele eye lobe ectoderm, ilo inner optic lobe, olo outer optic lobe
As a further test of N signaling activation we cloned part of the grasshopper ortholog of Dl (Sa_Dl) and examined its expression in the eye lobe. As for fng, no expression of Sa_Dl is detected in the eye lobe ectoderm before initiation of the morphogenetic furrow (not shown). In the differentiating retina, Sa_Dl is expressed in a tight band of cells in the morphogenetic furrow and in the photoreceptor cell clusters starting approximately six to seven cell diameters away from the furrow (Fig. 4f). No expression could be detected in the anterior grasshopper eye field unlike in Drosophila, where Dl is expressed in the dorsal eye field in front and posterior of the morphogenetic furrow (Cho and Choi 1998). Thus consistent with the results from assaying fng expression, the spatial regulation of Sa_Dl provides no evidence for N signaling activation along the midline in undifferentiated grasshopper retina field.
Grasshopper retina determination based on expression of eya and so orthologs
Specification of the Drosophila retina is established by co-expression of the transcription factors eyeless (ey; Quiring et al. 1994), eyes absent (eya; Bonini et al. 1993), sine oculis (so; Cheyette et al. 1994; Serikaku and O’Tousa 1994) and dachshund (dac; Mardon et al. 1994; for review, see Pappu and Mardon 2002). Postembryonic determination and differentiation of the Drosophila retina primordium is associated with dynamic regulation of the expression of these retinal determination (RD) genes (Bessa et al. 2002; Pappu and Mardon 2002). Wg, in antagonistic interaction with the signaling factors Dpp and Hh, which promote RD gene expression, plays a critical role in delimiting the anterior Drosophila eye field by inhibiting the expression of some of the RD genes (Baonza and Freeman 2002; Bessa et al. 2002). While the expression of ey and wg overlap in the anterior eye disc of the third larval instar, wg represses so, eya and probably dac in this region leading to non-overlapping expression patterns (Baonza and Freeman 2002). To investigate whether this regulatory relationship also exists in the grasshopper, we studied the expression of the grasshopper orthologs of so (Sa_so) and eya (Sa_eya) in relation to grasshopper wg.
Consistent with a shared role in visual primordium specification, both genes are expressed in very similar, overlapping expression domains in the developing visual system including the eye lobe ectoderm, outer optic lobe anlagen and ocelli (Fig. 5a, b, e, f). At about 25% of development, Sa_so and Sa_eya are expressed in a four- to seven-cell-wide margin that outlines almost the entire head lobe periphery including the lateral anlagen of the presumptive compound eye primordium (Fig. 5a, e). Expression is absent from the procephalic tissue anterior of the stomodeum most likely corresponding to the anlage of the labrum. In addition, expression levels decrease in a small region between the lateral protocerebrum and the visual primordium. Comparison with the procephalic wg expression domains at equivalent stages of development suggests that the peripheral-most border of the procephalic expression domain abuts but does not overlap with that of Sa_eya and Sa_so in the visual system (compare Fig. 2c with Fig. 5a, e).
Molecular control of retina specification in the grasshopper eye based on expression of Sa_eyes absent and Sa_sine oculis. a, b, e, f Frontal view of grasshopper embryonic head. c, g Optical section of eye lobe from lateral perspective at the level of the outer optic lobe anlage and d, h at the level of the peripheral ectoderm. i–l Close-up views of developing eye lobes from frontolateral perspective labeled for Sa_eya (i, j) or (k, l) double-labeled with Sa_eya (dark blue) and Sa_wg (red). a, e Hollow arrow points at area of reduced or missing expression between the lateral protocerebrum and the visual primordium. d, h Black arrow indicates the morphogenetic furrow. i, k Red arrowhead points to indentation in the posterior eye lobe margin. k, l Arrow indicates the borders of protocerebral ectodermal Sa_wg expression domains. Notice the slight overlap of Sa_eya and Sa_wg anteriorly in k but non-overlapping signals in l. ant Antenna, ele eye lobe ectoderm, elo eye lobe, ilo inner optic lobe, lbr labrum, man mandible, oce ocelli, olo outer optic lobe, sto stomodeum
After completion of head compartment morphogenesis at 40% of development, Sa_so and Sa_eya remain strongly co-expressed in the eye lobe ectoderm and outer optic lobe anlagen (Fig. 5c, g). In the eye lobe ectoderm, expression is seen throughout the differentiating retina, in the morphogenetic furrow and a wide region extending from the furrow (Fig. 5d, h). The expression domain in the anterior undifferentiated eye field is widest at the eye lobe poles and conspicuously narrower at the eye lobe midline. The gradient-like decrease of expression anterior of the morphogenetic furrow suggests that Sa_so and Sa_eya expression in this domain is most strongly determined by activating signaling factors emanating from the morphogenetic furrow and the polar margins.
At the polar margins of the eye lobe ectoderm, Sa_so and Sa_eya expression drops relatively sharply in front of the adjacent head ectoderm (Fig. 5c, g). This is particularly pronounced for Sa_eya at the dorsal pole (Fig. 5c). Different from the situation in the early procephalon, Sa_so and Sa_eya have cleared in the dorsal protocerebral ectoderm bordering to the eye lobe. Inspection of intermediate developmental stages confirms the gradual reduction of Sa_eya in the peripheral head epidermis anterior of the dorsal eye lobe pole (Fig. 5i, j). However, Sa_eya expression still seems to be present in the peripheral head epidermis at 27% of development when the dorsal ectodermal wg expression domain has been established indicating potential overlap (Fig. 5i). The relationship between Sa_wg and Sa_eya expression was further analyzed by double labeling in situ hybridization. These experiments confirm an initial overlap of ectodermal Sa_eya with Sa_wg during the early phase of eye lobe morphogenesis (Fig. 5k). At later stages, when the dorsal ectodermal protocerebral Sa_wg domain becomes incorporated into the eye lobe, Sa_eya and Sa_wg expression appear mutually exclusive at both the dorsal and ventral eye lobe poles (Fig. 5l). In combination, these results are consistent with Sa_wg repressing Sa_so and Sa_eya in the grasshopper eye primordium as their expression patterns are largely non-overlapping. The closely adjacent borders of Sa_wg and the retinal determination genes in the early and late grasshopper head imply that high levels of Wg signaling may be required for retinal determination gene repression. This may also explain the transient overlap of Sa_wg and retina determination gene expression in the protocerebral dorsal ectoderm.
Molecular control of eye field specification based on Exd expression
Wg cooperates with the TALE class homeodomain protein-encoding homothorax (hth) gene in patterning the border between retina and head cuticle in the Drosophila eye disc (Pai et al. 1998; Rieckhof et al. 1997). Like wg, hth is initially expressed throughout the entire Drosophila eye-antennal imaginal disc (Pichaud and Casares 2000) and expression clears from the posterior margin starting from the second larval instar due to repression by Dpp (Bessa et al. 2002). Normal hth expression levels require Wg signaling and Hth is essential for maintenance of wg expression in the ventral part of the eye imaginal disc (Pichaud and Casares 2000). Hth participates in a transcription factor complex consisting of Ey and the zinc finger transcription factor Teashirt (Tsh), which represses eya and dac in the anterior eye imaginal disc (Bessa et al. 2002). Hth thus engages in a positive regulatory feedback loop with wg in this context and is likely to partially mediate the repressive effect of wg on retina differentiation and RD gene expression (Pichaud and Casares 2000; Baonza and Freeman 2002).
To investigate if the involvement of hth in front of the developing retina field is conserved in grasshopper, we assayed Hth activity by virtue of its physical interaction with the essential cofactor homeodomain protein Extradenticle (Jaw et al. 2000). The availability of antibodies detecting Exd across species has been a powerful tool for elucidating the role of these selector proteins during appendage patterning (Abzhanov and Kaufman 2000; Gonzalez-Crespo and Morata 1996). In Drosophila, expression of cytosolic Exd is uniform while that of Hth is spatially controlled (Pai et al. 1998; Rieckhof et al. 1997). Hth binding to Exd leads to translocation of cytosolic Exd into the nucleus (Jaw et al. 2000; Rieckhof et al. 1997). Hth/Exd controlled tissue fields are thus marked by nuclear localized Exd protein. Consistent with this, Hth and nuclear Exd expression coincide in the Drosophila eye antennal imaginal disc (Pai et al. 1998; Pichaud and Casares 2000; personal observation).
The spatial regulation of Exd protein expression in the grasshopper embryonic head was examined between 25% and 45% of development. First expression was found in the early antennal appendages and the developing labrum (Fig. 6a). Throughout later development nuclear Exd remained prominent in the labrum and in the proximal third of the antenna consistent with its conserved function in proximal appendage patterning (Gonzalez-Crespo and Morata 1996; Fig. 6b–d). Unexpectedly, no additional regions of nuclear Exd could be observed in the developing head. Most importantly, no change of expression levels was detected at the anterior polar eye lobe borders neither before nor after initiation of retina differentiation (Fig. 6c). The lack of overlapping protocerebral Exd and wg expression domains and of non-appendage head Exd expression domains in general demonstrates that the molecular control of Drosophila head versus retina epithelium determination is not conserved in the grasshopper.
Expression of Extradenticle (Exd) in the developing grasshopper head. a–d Projections of confocal sections taken from frontal perspective of grasshopper embryonic heads labeled with α-Exd antibody (Aspland and White 1997). ant Antenna, ele eye lobe ectoderm, lbr labrum, olo outer optic lobe
Li inhibits furrow progression in the grasshopper embryonic eye lobe
The presence of strong wg expression domains in front of the developing eye field and the non-overlapping expression patterns of the RD genes Sa_eya and Sa_so at later stages of eye lobe development are consistent with a repressive effect of Wg signaling on retina differentiation. To test this hypothesis further, we investigated the effect of culturing grasshopper eye primordia in the presence of lithium, which has been used as a potent, across species activator of the Wg signaling pathway by blocking GSK-3β (Davies et al. 2000; Stambolic et al. 1996). In the Drosophila eye, genetic removal of the GSK-3β ortholog shaggy (sgg) inhibits photoreceptor differentiation while inducing expression of a constitutively active sgg results in ectopic furrow initiation (Hazelett et al. 1998; Treisman and Rubin 1995). These data suggest that GSK-3β functions as repressor of Wg signaling in the Drosophila retina.
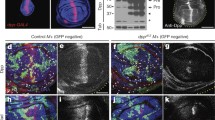
The development of in vitro cultured grasshopper eye lobes was analyzed in the presence and absence of 40 mM LiCl. In eye lobes incubated for 48 h without perturbation, the differentiating eye field dramatically expanded compared to reference eye lobes assayed at the beginning of the experiment (Fig. 7a). In contrast, the differentiating retina of eye lobes cultured in the presence of LiCl did not show any size increase compared to reference eye lobes indicating an immediate and complete stall of furrow progression (Fig. 7a). Furthermore, the array of ommatidia forming cell groups was disordered and the morphology of the furrow appeared abnormal. No difference in retina field size increment or morphology was observed in eye lobes challenged with 40 mM NaCl suggesting that the inhibition of furrow progression in LiCl-treated tissue was specifically due to the increase in Li concentration (Fig. 7a).
The effect of Li on grasshopper eye development. a Retina differentiation in cultured eye lobes. Projections of confocal image stacks taken from phalloidin-labeled tissue. The 0 h reference eye lobes of culture experiments started at 40% of development. Morphogenetic furrow indicated by arrow. b The impact of Li on apoptosis and mitosis in cultured eye lobes. Upper panels show the comparison of mitotic cell distribution and density in Li-treated and control tissue. Arrowheads point at mitotic cells. Note the increased density of mitotic cells in Li-treated eye lobe, especially anterior to the morphogenetic furrow (arrow). Lower panels show the comparison of apoptotic cell distribution and density. Dotted circles highlight apoptotic cells. c Descriptive statistics of apoptotic cell frequency in Li-treated and control eye lobes anterior and posterior to morphogenetic furrow. Error bars indicate standard error based on five samples per group. d Descriptive statistics of mitotic cell frequency presented as in c
To determine if the stall in furrow progression was a secondary effect of induction of cell death or inhibition of cell proliferation, we investigated distribution and density of apoptotic and mitotic cells in cultured eye lobes. In tissue labeled with the DNA dye propidium iodide apoptotic cells can be identified as carrying a homogenous, highly condensed mass of chromosomal material whereas mitotic cells are characterized by the presence of regularly condensed chromosomes (Fig. 7b). Differential effects of Li treatment on cell death and proliferation were observed along the anteroposterior axis of the developing retina field (Fig. 7c, d). Apoptotic cell density increased only marginally in the undifferentiated epithelium anterior to the morphogenetic furrow, but dramatically in the differentiating retina posterior to the furrow (Fig. 7c). This pattern parallels the apoptotic effect of Wg signaling on differentiated photoreceptors (Ahmed et al. 1998; Freeman and Bienz 2001). We did not determine whether the cell death observed in the grasshopper differentiating retina was specific to photoreceptors. In contrast to cell death, mitotic cell density was strongly increased anterior to the furrow but not posterior to it (Fig. 7d). In combination, these results suggest that the Li-induced furrow stall phenotype is not the result of eye field wide changes in cell proliferation or apoptosis. On the contrary, Li stimulates cell proliferation anterior to the furrow which might be expected to enhance rather than reduce furrow progression. The increase of apoptosis posterior of the morphogenetic furrow is significant yet far from approaching uniform cell death (Fig. 7b). The stall of furrow progression is thus unlikely a direct consequence of increased cell death. The immediacy of furrow block in response to Li furthermore argues against reduced levels of furrow progression promoting factors as possible cause. Overall, the effect of Li on grasshopper retina differentiation is very similar to that of manipulating GSK-3β or Wg signaling activity in the Drosophila eye disc suggesting that the role of wg in inhibiting retina differentiation and promoting tissue proliferation is conserved in the grasshopper.
Discussion
We show here that the expression of the signaling factor wg and genes which interact with wg in the Drosophila eye exhibit similarities but also significant differences in the developing embryonic eye primordium of the grasshopper Schistocerca americana. The similarities are informative with regards to patterning mechanisms regulating embryonic eye development in primitive insects. The differences highlight unexpected developmental regulatory changes most likely resulting from the evolutionary transition from embryonic to postembryonic eye development in higher insects. In the following, we discuss the developmental organization of the early grasshopper eye primordium and the evolution of developmental mechanisms in the insect eye.
Homology relationships of wg expression domains in the developing grasshopper visual system
Examination of wg expression in the grasshopper embryonic procephalon reveals eye primordium-related expression domains in three different tissue types: neuroectoderm, ectoderm and neuroblasts of the outer optic lobe anlage. In each of these tissues, wg is expressed in pairs of domains which relate to the dorsoventral axis of the eye field in the adult head capsule. Despite significant differences, all three domains can be related to domains in Drosophila based on similarities in spatiotemporal expression control.
Outer optic lobe anlagen domains
The pair of optic lobe anlagen cell clusters in the grasshopper embryonic head corresponds to the bilateral wg expression domains in the anlagen of the Drosophila outer optic lobe (Kaphingst and Kunes 1994). In both species, expression initiates in a single or very few cells, expands to form expression clusters during subsequent development, and persists during optic lobe development (Friedrich and Benzer 2000; Kaphingst and Kunes 1994). In Drosophila, wg is expressed slightly earlier at the ventral pole of the optic lobe anlage than in the dorsal pole (Kaphingst and Kunes 1994). We did not observe differential timing in Schistocerca but cannot exclude this possibility. The Drosophila outer optic lobe wg domains are involved in stimulating tissue growth (Kaphingst and Kunes 1994). Considering the early onset of wg expression in the grasshopper optic lobe anlagen, and the subsequent massive growth of the latter, it is reasonable to assume that this function is conserved. This is further supported by the fact that the early outer optic lobe exhibits a conspicuous bilobed structure leading to an indentation at the midline in the posterior margin (see Figs. 4b, 5i, k). This is suggestive of faster size increase at the poles of the anlagen consistent with the closer proximity of polar tissue to the Wg-expressing cells. The Drosophila outer optic lobe domains also function as instructional cues of dorsoventral polarity by inducing target genes such as Dll, omb and dpp in nested bilateral symmetric expression domains. Preliminary observations suggest that these regulatory relationships are not conserved because dpp and Dll expression cannot be detected in the grasshopper outer optic lobes (Friedrich, personal observation).
Protocerebral ectodermal domains
The protocerebral ectodermal wg domains correspond to the polar wg expression domains in the anterior field of the third instar Drosophila eye disc (Fig. 8a). The location of the dorsal domain strongly indicates that it is, as in Drosophila, associated with anlagen of head epidermis closely adjacent to the retina. However, preliminary comparisons of head epidermis landmarks suggest that it is less inclusive than its Drosophila counterpart (Friedrich 2003). Interestingly, the dorsal grasshopper ectodermal domain integrates into the eye lobes during the second half of development. This suggests that the maturing eye lobe includes both precursor tissue of the retina and of the adjacent head epidermis.
Schematic summary of wingless expression and function in the developing grasshopper eye. a Comparison of wg expression domains in the second and late third instar eye imaginal disc of Drosophila and the grasshopper eye lobe at 30% and 45% of development. b Model of Wg patterning functions in the early grasshopper eye lobe. Wg diffusing from the dorsal and ventral protocerebral expression domains stimulates mitosis (M) at short distance, and represses retina differentiation (D) at long distance in the eye lobe ectoderm. Specific Wg expression domains also activate tissue proliferation in the polar margins of the developing outer optic lobe. Polar tissue proliferation leads to the bilobed outline of the early grasshopper eye lobe. Blue domains represent hypothetical Wg signaling gradients. DOA dorsal optic lobe anlagen domain, DPN dorsal protocerebral neuroectoderm domain, DPE dorsal protocerebral ectoderm domain, ele eye lobe ectoderm, MPN median protocerebral neuroectoderm domain, olo outer optic lobe, VOA ventral optic lobe anlagen domain, VPE ventral protocerebral ectoderm domain, VPN ventral protocerebral neuroectoderm domain
The ectodermal domains contribute to the fine margin of wg-expressing cells surrounding the retina field during the late second half of embryogenesis which is derived from both the ectodermal and neuroectodermal expression domains. In later stages, this frame begins to surround the entire eye field supplemented by additional expression in the basal membrane (not shown). Besides potentially delimiting retina differentiation at the anterior margin of the retinal field, this expression aspect is most likely involved in patterning the eye field margin during terminal differentiation as has been found in Drosophila (Lin et al. 2004; Tomlinson 2003; Wernet et al. 2003). It would be interesting to investigate if the occurrence and removal of peripheral ommatidia with aberrant numbers of photoreceptors is conserved (Lin et al. 2004). The formation of similar eye field margin wg expression domains in the flour beetle Tribolium castaneum and the crustacean Mysidium columbiae indicates that this patterning aspect is ancient (Duman-Scheel et al. 2002a; Friedrich and Benzer 2000).
A further notable similarity of the ectodermal expression domains between fly and grasshopper is that the dorsal domain is activated earlier than the ventral domain. The course of dorsal domain formation is, however, very different in both species. In Drosophila, the dorsal expression domain is derived by repression of the posterior half of an originally larger wg domain which extends through the entire dorsal compartment of the retina primordium. In the grasshopper, the dorsal ectodermal expression domain is initiated outside of the anlage of the visual primordium. This difference correlates with the lack of dpp in the early grasshopper eye lobe (Friedrich and Benzer 2000), which represses wg from the posterior margin in the Drosophila eye disc (Chanut and Heberlein 1997; Pignoni and Zipursky 1997; Royet and Finkelstein 1997; Wiersdorff et al. 1996).
Protocerebral neuroectodermal domains
The evolutionary relationships of the protocerebral neuroectodermal domains to head-patterning-related wg expression in Drosophila are more difficult to resolve. Similarities exist with both embryonic and postembryonic Drosophila wg expression domains. The origin of the grasshopper domains from the primordial procephalic wg domains links them to the procephalic wg expression domains in the Drosophila embryo which derive in a similar way from the anterior-most “rostral” expression domain in the blastoderm embryo (Baker 1988a; personal observation). A second shared feature is the contribution of neuroblasts to the embryonic protocerebrum by these domains (Richter et al. 1998). However, unlike in the grasshopper, the Drosophila neuroectodermal domains seem to persist as undivided head lobe domains (Baker 1988a). Comparative analysis of wg expression in the embryo of the flour beetle Tribolium castaneum, a primitive holometabolous insect, suggests that this situation in Drosophila is derived (Nagy and Carroll 1994; personal observation) further supporting that the grasshopper and Drosophila procephalic neuroectodermal wg domains share evolutionary ancestry. However, the dorsal and ventral grasshopper protocerebral neuroectoderm domains also share characteristics with the polar wg domains in the Drosophila third instar eye disc. Firstly, the initially neuroectodermal grasshopper domains are retained as ectodermal domains after conclusion of brain formation eventually integrating into the margin of wg expression ectoderm around the retina field. Secondly, until this stage, their strategic location as polar Wg sources in front of the developing retina is compatible with patterning functions of the similarly positioned ectodermal domains in the Drosophila eye disc. The grasshopper protocerebral neuroectoderm wg-expressing cells may thus be driven by regulatory elements which have been inherited in part by embryonic and in part by postembryonic wg-expressing cells in Drosophila. It seems that integrated patterning aspects of ancestral head development still conserved in the grasshopper became decoupled during the evolution of complete metamorphosis in Drosophila.
Remarkably, very similar procephalic wg expression domains have been detected in the developing embryonic head of the centipede Lithobius atkinsoni (Hughes and Kaufman 2002). Considering the current view of arthropod phylogeny (Hwang et al. 2001), this implies that the expression of wg as described for the grasshopper reflects aspects of head patterning in the ancestor of insects, crustaceans and myriapods and thus, most likely, of all arthropods. Partial reduction of procephalic wg expression domains seems to have occurred during the evolution of spiders and in the lineage leading to the millipede Glomeris marginata (Damen 2002; Janssen et al. 2004).
Wg and axis formation in the grasshopper embryonic eye field
Earlier investigations of wg expression in the developing crustacean visual system noted the conserved expression in polar domains in front of the developing retina (Duman-Scheel et al. 2002a). From similar observations in the grasshopper, it was concluded that associated wg patterning functions in the Drosophila eye disc, including the spatial coordination of furrow initiation at the midline point of the posterior eye field margin, are conserved (Friedrich and Benzer 2000). This makes wg a conserved determinant of the planar axes in the insect eye field. However, considering the complexity of the protocerebral wg expression in grasshopper, its possible role in setting up the embryonic eye field of primitive insects deserves further examination.
In the Drosophila eye, wg-controlled axis specification is based on two basic elements: the inhibitory effect of wg on photoreceptor differentiation and the strategic position of the polar ectodermal eye disc expression domains in front of the furrow. Evidence that Wg signaling also inhibits photoreceptor differentiation in the grasshopper retina primordium comes from the observation that Li specifically blocks furrow progression in cultured eye lobes (Fig. 7). Li inhibits the constitutive Wg signaling repressor GSK-3β (Stambolic et al. 1996). The effect observed, stalling of the furrow, is therefore consistent with that predicted from a repressive function of Wg signaling on retina differentiation. A caveat in the interpretation of this result is that GSK-3β, the direct target of LiCl, also participates in signaling pathways other than canonical Wnt signaling. Most importantly, GSK-3β has been shown to function as a repressive component in the Hh signaling pathway (Jia et al. 2002). In contrast to wg, hh is an essential activator of furrow progression. Hh also stimulates cell proliferation posterior to the morphogenetic furrow (Duman-Scheel et al. 2002b). The immediate stall of furrow progression and the specific enhancement of cell division anterior to the furrow in response to LiCl treatment in grasshopper suggests that the effect of repressing GSK-3β on the Wg signaling pathway overrides that on Hh signaling. This is consistent with the GSK-3β phenotypes in the Drosophila eye which are Wg-signaling-specific as well (Hazelett et al. 1998). It is therefore reasonable to conclude that the effect of Li on furrow progression in cultured grasshopper eye lobes is an indicator of inhibition of retina differentiation by Wg signaling.
The position of the protocerebral ectoderm and neuroectoderm wg domains in front of the grasshopper embryonic eye field is compatible with forcing the initiation of the furrow to the posterior margin of the eye field. However, the spatial regulation of furrow initiation in Drosophila is mediated by ectodermal wg expression which initially reaches into the presumptive initiation point and subsequently retracts but persists close to the front of the progressing morphogenetic wave (Cho et al. 2000; Fig. 1). The situation in grasshopper appears different in several respects. The protocerebral ectoderm domains emerge relatively late and at a considerable distance from the initiating furrow (Fig. 8a). At the time of furrow initiation, the dorsal ectoderm domain is strong while expression levels in the ventral ectoderm are hardly detectable (Fig. 2f). This suggests that the neuroectoderm domains, which emerge earlier and are positioned closer to the eye field midline, are a major determinant of early wg-mediated eye field patterning. As in Drosophila, they are present at the beginning of grasshopper retina primordium formation and may instruct the anterior pole of the eye field by maximal signaling levels and the midline as expression levels sink along the dorsoventral axis of the eye (Fig. 8b). However, these domains are at a considerable distance from the initiation point. Secondly, their position is more median in the eye field implying a shallower protein gradient towards the midline assuming similar protein diffusion mechanisms. Since the neuroectoderm domains are also involved in neurogenesis, a function in epidermal patterning is not essential to explain their presence. Further evidence for a conserved wg function in spatial regulation of furrow initiation comes from the observation in fng-labeled tissues that the initiation point of the furrow is slightly off the eye lobe midline, shifted towards the ventral margin (Fig. 4b). This correlates with the larger size of both the ectodermal and neuroectodermal wg domains at the dorsal pole compared to the ventral pole (Fig. 2e, f).
Localized stimulation of cell proliferation may be a synergistic component of eye field patterning by wg. Mutant phenotypes of wg and Wg signaling components such as axin demonstrated that Wg signaling is an important activator of growth in the early eye disc and later in the anterior eye disc field (Baonza and Freeman 2002; Lee and Treisman 2001; Treisman and Rubin 1995). Early reduction of wg signaling leads to smaller eyes (Kaphingst and Kunes 1994; Ma and Moses 1995) as opposed to late reduction which leads to ectopic retina (Ma and Moses 1995; Treisman and Rubin 1995). Consistent with a conserved function of wg in stimulating growth of the early eye field, the mitotic cell frequency is specifically increased in the anterior eye field of Li-treated grasshopper retinae (Fig. 7d). Interestingly, the early grasshopper eye lobes are distinctly bi-lobed. The polar margins appear larger and the ectoderm in these regions thicker. Very often a slight indentation can be observed at the midline point in the posterior margin where the ectoderm remains thin (see Fig. 5i, k). A similar bi-lobed morphology is observed in the early developing outer optic lobe anlagen where wg is likely to stimulate growth as well (see above). In combination, these data are compatible with Wg-stimulated tissue growth in both the lateral eye lobe ectoderm and the outer optic lobe. The organizing function of wg in grasshopper eye field axis formation may result from the combined effect of short range stimulation of tissue growth and long range inhibition of retina differentiation (Fig. 8b).
Dorsoventral compartment formation in the grasshopper retina
The Drosophila eye is a striking example of planar cell polarity patterning and tissue compartmentalization. In the dorsal retina hemisphere the initially anterior-most R3/R4 photoreceptor cell pair of each ommatidium points towards the dorsal pole after a 90° rotation of the developing ommatidium. The same patterning event results in a mirror image orientation of R3/R4 cells in the ventral hemisphere thus pointing towards the ventral pole. The border between these two ommatidial compartments defines the equator running very precisely along the retina field midline (Dietrich 1909; Ready et al. 1976). The dorsal compartment is further partitioned into normal retina and the narrow margin of dorsal rim ommatidia specialized for the detection of polarized light (Wada 1974).
Midline-related as well as dorsal rim area compartments exist in many insect species, yet there are also many lacking either one or both. The grasshopper falls into the class of insects that possess a dorsal rim area but lack morphological or physiological evidence for midline-related eye field compartments. Unlike in Drosophila, the developing ommatidia do not rotate in grasshopper (Friedrich, unpublished). Two ommatidial variants exist, which differ by the position of the R8 cell. They are, however, randomly distributed across the retina (Wilson et al. 1978). An approximately 450-ommatidia-large dorsal rim area fills the dorsal-most pole of the grasshopper eye field (Homberg and Paech 2002).
Analyzing the expression of genes interacting with wg during dorsoventral compartment formation in Drosophila provides the opportunity to investigate if dorsoventral compartment formation is a general building principle of insect eye development or specific to insects in which dorsoventral compartments are morphologically or physiologically expressed. To this end we have investigated the expression of the homologs of pnr and Iro-C genes in the grasshopper. In Drosophila, pnr is expressed in late embryonic stages in the dorsal cells of the eye primordium (Maurel-Zaffran and Treisman 2000). Although there is a transient decline of pnr expression in the early first instar eye disc (Singh and Choi 2003), pnr expression can be detected along the dorsal margin in the second instar eye disc long before the onset of retina differentiation. This expression persists into the third instar disc where it is restricted to the anterior dorsal edge and overlaps with wg (Maurel-Zaffran and Treisman 2000; Singh and Choi 2003). The expression of the Iro-C dorsal selector genes is initiated during the early second instar. Their expression expands through the dorsal half of the eye disc and persists into the undifferentiated eye field of the third instar eye disc (McNeill et al. 1997; Singh and Choi 2003; Fig. 9a).
Schematic comparison of the expression genes interacting with wingless in Drosophila and grasshopper. a Comparison of genes involved in dorsoventral compartment formation and Notch-signaling organizer formation. b Comparison of the genes involved in the regulation of eye specification and head epidermis patterning
The expression of wg, pnr and Iro-C homologs in grasshopper indicates that the partitioning of the retina into dorsoventral compartments differs from Drosophila not only at the morphological but also at the molecular developmental level (Fig. 9a). (1) Pnr expression does not reach into the retina field like in Drosophila. (2) Pnr expression does not overlap with wg. This finding precludes dorsal activation of wg by pnr. Interestingly, wg is also expressed before pnr in the Drosophila eye disc (Cho et al. 2000; Singh and Choi 2003). (3) Although the dorsal ectodermal expression domain of wg forms early in both Schistocerca and Drosophila, it does not extend throughout the entire dorsal half of the grasshopper eye field as in Drosophila (Figs. 2, 8a). In fact, Sa_wg is never expressed in cells that contribute to the eye primordium proper but adjacent to the dorsal anterior eye field. This raises the possibility of gene regulation in the grasshopper retina by Wg diffusing into the dorsal margin, which is supported by expression of Sa_iro in the dorsal-most cap of the embryonic grasshopper eye field. (4) As in the case of wg, the expression domain of Sa_iro does not extend far away from the dorsal pole. It is therefore unlikely to be involved in a midline-related compartment formation process like in the early Drosophila eye disc.
While the grasshopper retina appears to lack midline-related dorsoventral compartments, the expression data are consistent with a molecular genetic control of dorsal rim area specification similar to Drosophila. In Drosophila, caup and ara gene expression reinitiates during pupation and persists into the adult (Wernet et al. 2003). Their expression is essential for the specification of the dorsal rim ommatidia in combination with wg emanating from the retina periphery (Wernet et al. 2003). There is a strong spatial correlation between the dorsally strongly restricted Sa_iro expression in the embryonic grasshopper eye field and the dorsally restricted outline of the dorsal rim area in first instar grasshopper nymphs (not shown). In addition to dorsally localized Sa_iro, Sa_wg is expressed in the head cuticle bordering the retina as in Drosophila. Assuming persistence of these expression domains into later development, the grasshopper dorsal rim ommatidia very likely experience the same signaling factor and selector gene input as in Drosophila.
In summary, these data indicate similar dorsoventral retina compartment patterning in grasshopper and fruit flies involving wg and Iro-C genes. It is most parsimonious to assume that induction of Iro-C genes by wg is generally involved in insect dorsal rim area patterning. This, however, does not necessarily reject the view that the widespread yet scattered occurrence of dorsal rim ommatidia in insects is the result of parallel evolution (Labhart and Meyer 1999). Our data also identify pnr and Iro-C genes as conserved regulators of dorsal body wall specification in insects. It is possible that elements of the conserved dorsoventral body wall specification gene network were recruited for the retina patterning at multiple times during evolution. This would represent examples of convergent evolution of morphological traits via recruitment of identical gene networks as recently demonstrated in closely related Drosophila species (Gompel and Carroll 2003; Sucena et al. 2003). One possibility to test this hypothesis further will be to examine the expression of Drosophila downstream mediators of dorsal rim photoreceptor specification such as hth in other species (Wernet et al. 2003). Shared employment of hth would strengthen the case for homology of dorsal rim ommatidia in different insects while absence of hth expression in developing dorsal rim ommatidia of non-dipteran species would favor convergent evolution. Similar arguments may be made regarding the evolution of the taxonomically widely scattered occurrence of morphological equators in insect and crustacean retinas. Denser taxon sampling will be required to discriminate cases of parallel evolution and homology.
It is notable that dorsal wg in the early Drosophila eye disc has been described as being predominantly in the peripodial membrane layer (Cho et al. 2000). A possible functional equivalent to the peripodial membrane is the amnionic membrane which intimately covers the eye lobe ectoderm until 40% of development (Friedrich and Benzer 2000). We were, however, unable to detect wg in cells of the amnionic membrane (not shown). The peripodial membrane as a morphogenetic unit is specific to higher flies. It is possible that the derived function of wg in equator formation is correlated with the evolution of the peripodial membrane. The conserved dorsal rim area specification step on the other hand occurs in a two-dimensional context similar in the grasshopper embryo and Drosophila pupal eye.
Insect eye development in the absence of a N signaling organizer
The absence of midline-related dorsoventral compartment formation is most surprising considering that the equator functions as the essential organizer of Drosophila eye disc development. Analogous to the situation in the Drosophila wing disc, it is the center of N signaling activity which promotes growth, polarization and differentiation of the eye field (Cavodeassi et al. 1999; Cho and Choi 1998; Dominguez and de Celis 1998; Papayannopoulos et al. 1998). The specific activation of N signaling along the eye field midline is mediated by the dorsal-compartment-specific expression of Dl and ventral-specific expression of Ser and fng. If any of these components is missing, such that N signaling is abrogated, the Drosophila eye disc fails to develop. Likewise, ectopic induction of dorsoventral gene expression borders induces ectopic N signaling and ectopic induction of morphogenetic furrows.
The comparison of the expression of genes involved in dorsoventral compartment formation and N signaling between Drosophila and grasshopper strongly suggests that the grasshopper retina primordium develops in the absence of a N signaling organizer (Fig. 9a). The expression of dorsal compartment specification genes does not extend to the midline. Consistent with this, the N signaling components fng and Dl, which exhibit dorsoventral-compartment-specific expression patterns in the undifferentiated anterior eye field of the Drosophila retina, show conserved expression in the morphogenetic furrow but not equator-related expression in front of the furrow. The lack of N involvement in the early grasshopper eye field thus marks a major difference between grasshopper and Drosophila eye development. It will be interesting to investigate if the situation observed in grasshopper is representative for primitive insects in general. It is tempting to speculate that the N signaling organizer was evolutionarily recruited as a module to enhance the rapid and continuous postembryonic development of the Drosophila eye imaginal disc. Further support for this possibility comes from the fact that, like dorsal wg, some components of the N organizer network, including fng and Ser, are expressed in the evolutionarily derived peripodial membrane (Cho et al. 2000; Gibson and Schubiger 2000).
Evolutionary divergence of head epidermis patterning
Analogous to its role in regional specification during appendage development, wg participates in shaping the border between retina and adjacent head cuticle in the Drosophila eye imaginal disc (Royet and Finkelstein 1997). This seems to be its main function after initiation of the morphogenetic furrow. At this stage wg expression fills part of the dorsal and ventral anterior margin of the eye disc region which corresponds to presumptive head cuticle compartments. The similarity of polar neuroectodermal and ectodermal expression domains in front of the grasshopper embryonic eye primordia suggests that this wg patterning function is conserved in the grasshopper.
Further support comes from the finding of largely non-overlapping expression patterns between the retina determination genes Sa_eya and Sa_so throughout the posterior retinal field, and Sa_wg expressed in front of the eye field. The anterior border of their expression domains is closely abutting with that of wg which would suggest that Wg is a weak repressor of Sa_eya and Sa_so expression. Consistent with this, Sa_eya and Sa_so expression appears reduced but not abolished in cultured grasshopper eye lobes treated with LiCl (not shown). The area of transient overlapping expression of Sa_wg and the retinal determination genes in the dorsal embryonic ectoderm maps to a field of cells which does not contribute to eye primordium. This change may reflect the progressive compartmentalization of the grasshopper head where the selector genes so and eya seem to initially be expressed in wider domains than the actual anlagen of the eye primordia (Fig. 5a, e).
However, it is surprising to find that Exd, which together with hth cooperates with wg during Drosophila head patterning, is not expressed in the grasshopper protocerebral head epidermis including the eye lobe margin (Fig. 9b). Drosophila eye disc tissue clones mutant for either hth or exd cause ectopic eye formation in the ventral head while ectopic expression of hth but not exd suppresses retina differentiation (Casares and Mann 1998; Pai et al. 1998; Pichaud and Casares 2000). Nonetheless, no nuclear expression of Exd can be detected in the grasshopper dorsal embryonic head implying lack of hth expression. Consistent results can be noted in Exd-stained specimens of the house cricket Gryllus domestica, an orthopteran distantly related to grasshopper (Abzhanov and Kaufman 2000). Furthermore, RNAi knockdown of hth in another hemimetabolous insect, the milkweed bug Oncopeltus fasciatus, results in strong appendage phenotypes but the head region develops without noticeable defects (Angelini and Kaufman 2004). Pending absence of yet unidentified hth paralogs, these data strongly suggest that Exd/Hth does not participate in patterning of the procephalic head epidermis in primitive insects. One must therefore conclude that the molecular genetics of head versus eye compartment allocation in primitive insects differs from that in the Drosophila eye disc.
In this context, it is notable that the lack of Exd/Hth at the eye field border correlates with lack of dpp expression in the grasshopper morphogenetic furrow (Friedrich and Benzer 2000). In Drosophila, dpp promotes furrow movement primarily by repressing hth in cells immediately anterior to the furrow (Bessa et al. 2002). Vice versa, hth can suppress dpp and furrow movement if ectopically expressed (Pichaud and Casares 2000). This antagonistic relationship may be specific to the dynamic mode of head epidermis specification during Drosophila postembryonic head development.
Possible causes driving the developmental evolution of insect eye development
The present analysis reveals substantial differences in the molecular programs regulating early eye field growth and head versus eye primordium specification between grasshopper and Drosophila. This supplements the previous finding of divergent dpp expression patterns implying differences in the regulation of furrow initiation and progression (Friedrich and Benzer 2000). The final structure of the compound eye shows comparatively minor cell architectural differences between grasshopper and Drosophila. Consistent with this, the cellular dynamics of eye differentiation are highly conserved between the two species. It is therefore most likely that the differences in early developmental eye field organization and global patterning mechanisms are related to the shift of eye primordium formation from embryogenesis in primitive insects like grasshopper to postembryogenesis in Drosophila. The fruit fly represents a derived form of holometabolous development. All epidermal head structures develop de novo from imaginal discs. In more primitive holometabolous insects the eye develops directly from the lateral head epidermis of the larval head. The differences between Drosophila and grasshopper eye development may thus be related either to developmental changes that occurred during the early evolution of insect metamorphosis or during the later evolution of the Drosophila mode of postembryonic development from imaginal discs. This can be determined by extending the comparative analysis to primitive holometabolous species such as the flour beetle Tribolium castaneum or the tobacco horn moth Manduca sexta.
References
Abzhanov A, Kaufman TC (2000) Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol 227:673–689
Ahmed Y, Hayashi S, Levine A, Wieschaus E (1998) Regulation of armadillo by a Drosophila APC inhibits neuronal apoptosis during retinal development. Cell 93:1171–1182
Angelini DR, Kaufman TC (2004) Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects. Dev Biol 271:306–321
Aspland SE, White RA (1997) Nucleocytoplasmic localisation of extradenticle protein is spatially regulated throughout development in Drosophila. Development 124:741–747
Baker NE (1988a) Localization of transcripts from the wingless gene in whole Drosophila embryos. Development 103:289–298
Baker NE (1988b) Transcription of the segment-polarity gene wingless in the imaginal discs of Drosophila, and the phenotype of a pupal-lethal wg mutation. Development 102:489–497
Baker NE (2002) Notch and the patterning of ommatidial founder cells in the developing Drosophila eye. In: Moses K (ed) Drosophila eye development results and problems in cell differentiation 37. Springer-Verlag, Berlin Heidelberg New York, pp 5–19
Baonza A, Freeman M (2001) Notch signalling and the initiation of neural development in the Drosophila eye. Development 128:3889–3898
Baonza A, Freeman M (2002) Control of Drosophila eye specification by Wingless signalling. Development 129:5313–5322
Bentley D, Keshishian H, Shankland M, Toroian-Raymond A (1979) Quantitative staging of embryonic development of the grasshopper, Schistocerca nitens. J Embryol Exp Morphol 54:47–74
Bessa J, Gebelein B, Pichaud F, Casares F, Mann RS (2002) Combinatorial control of Drosophila eye development by eyeless, homothorax, and teashirt. Genes Dev 16:2415–2427
Bonini NM, Leiserson WM, Benzer S (1993) The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72:379–395
Boyan GS, Williams JL, Reichert H (1995) Morphogenetic reorganization of the brain during embryogenesis in the grasshopper. J Comp Neurol 361:429–440
Cadigan KM (2002) Wnt signaling—20 years and counting. Trends Genet 18:340–342
Cadigan KM, Nusse R (1996) Wingless signaling in the Drosophila eye and embryonic epidermis. Development 122:2801–2812
Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305
Cadigan KM, Jou AD, Nusse R (2002) Wingless blocks bristle formation and morphogenetic furrow progression in the eye through repression of Daughterless. Development 129:3393–3402
Calleja M, Herranz H, Estella C, Casal J, Lawrence P, Simpson P, Morata G (2000) Generation of medial and lateral dorsal body domains by the pannier gene of Drosophila. Development 127:3971–3980
Casares F, Mann RS (1998) Control of antennal versus leg development in Drosophila. Nature 392:723–726
Cavodeassi F, Diez Del Corral R, Campuzano S, Dominguez M (1999) Compartments and organising boundaries in the Drosophila eye: the role of the homeodomain Iroquois proteins. Development 126:4933–4942
Chanut F, Heberlein U (1997) Role of decapentaplegic in initiation and progression of the morphogenetic furrow in the developing Drosophila retina. Development 124:559–567
Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL (1994) The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12:977–996
Cho KO, Choi KW (1998) Fringe is essential for mirror symmetry and morphogenesis in the Drosophila eye. Nature 396:272–276
Cho KO, Chern J, Izaddoost S, Choi KW (2000) Novel signaling from the peripodial membrane is essential for eye disc patterning in Drosophila. Cell 103:331–342
Damen WG (2002) Parasegmental organization of the spider embryo implies that the parasegment is an evolutionary conserved entity in arthropod embryogenesis. Development 129:1239–1250
Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105
Dearden P, Akam M (2000) A role for Fringe in segment morphogenesis but not formation in the grasshopper, Schistocerca gregaria. Genes Dev Evol 210:329–336
Dearden PK, Akam M (2001) Early embryo patterning in the grasshopper, Schistocerca gregaria: wingless, decapentaplegic and caudal expression. Development 128:3435–3444
Dietrich W (1909) Die Facettenaugen der Dipteren. Z Wiss Zool 92:465–539
Dominguez M, de Celis JF (1998) A dorsal/ventral boundary established by Notch controls growth and polarity in the Drosophila eye. Nature 396:276–278
Dong Y, Dinan L, Friedrich M (2003) The effect of manipulating ecdysteroid signaling on embryonic eye development in the locust Schistocerca americana. Dev Genes Evol 213:587–600
Duman-Scheel M, Pirkl N, Patel NH (2002a) Analysis of the expression pattern of Mysidium columbiae wingless provides evidence for conserved mesodermal and retinal patterning processes among insects and crustaceans. Dev Genes Evol 212:114–123
Duman-Scheel M, Weng, L, Xin S, Du W (2002b) Hedgehog regulates cell growth and proliferation by inducing cyclin D and cyclin E. Nature 417:299–304
Fanto M, McNeill H (2004) Planar polarity from flies to vertebrates. J Cell Sci 117:527–533
Freeman M, Bienz M (2001) EGF receptor/Rolled MAP kinase signalling protects cells against activated Armadillo in the Drosophila eye. EMBO Rep 2:157–162
Friedrich M (2003) Evolution of insect eye development: first insights from fruit fly, grasshopper and flour beetle. Am Zool 43:508–521
Friedrich M, Benzer S (2000) Divergent decapentaplegic expression patterns in compound eye development and the evolution of insect metamorphosis. J Exp Zool 288:39–55
Gibson MC, Schubiger G (2000) Peripodial cells regulate proliferation and patterning of Drosophila imaginal discs. Cell 103:343–350
Gomez-Skarmeta JL, Diez del Corral R, de la Calle-Mustienes E, Ferre-Marco D, Modolell J (1996) Araucan and caupolican, two members of the novel iroquois complex, encode homeoproteins that control proneural and vein-forming genes. Cell 85:95–105
Gompel N, Carroll SB (2003) Genetic mechanisms and constraints governing the evolution of correlated traits in drosophilid flies. Nature 424:931–935
Gonzalez-Crespo S, Morata G (1996) Genetic evidence for the subdivision of the arthropod limb into coxopodite and telopodite. Development 122:3921–3928
Green P, Hartenstein AY, Hartenstein V (1993) The embryonic development of the Drosophila visual system. Cell Tissue Res 273:583–598
Haynie JL, Bryant PJ (1986) Development of the eye-antenna imaginal disc and morphogenesis of the adult head in Drosophila melanogaster. J Exp Zool 237:293–308
Hazelett DJ, Bourouis M, Walldorf U, Treisman JE (1998) Decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development 125:3741–3751
Heberlein U, Borod ER, Chanut FA (1998) Dorsoventral patterning in the Drosophila retina by wingless. Development 125:567–577
Heitzler P, Haenlin M, Ramain P, Calleja M, Simpson P (1996) A genetic analysis of pannier, a gene necessary for viability of dorsal tissues and bristle positioning in Drosophila. Genetics 143:1271–1286
Homberg U, Paech A (2002) Ultrastructure and orientation of ommatidia in the dorsal rim area of the locust compound eye. Arthropod Struct Dev 30:271–280
Hughes CL, Kaufman TC (2002) Exploring myriapod segmentation: the expression patterns of even-skipped, engrailed, and wingless in a centipede. Dev Biol 247:47–61
Hwang UW, Friedrich M, Tautz D, Park, CJ, Kim W (2001) Mitochondrial protein phylogeny joins myriapods with chelicerates. Nature 413:154–157
Irvine KD, Wieschaus E (1994) Fringe, a boundary-specific signaling molecule, mediates interactions between dorsal and ventral cells during Drosophila wing development. Cell 79:595–606
Janssen R, Prpic NM, Damen WG (2004) Gene expression suggests decoupled dorsal and ventral segmentation in the millipede Glomeris marginata (Myriapoda: Diplopoda). Dev Biol 268:89–104
Jaw TJ, You LR, Knoepfler PS, Yao LC, Pai CY, Tang CY, Chang LP, Berthelsen J, Blasi F, Kamps MP, Sun YH (2000) Direct interaction of two homeoproteins, homothorax and extradenticle, is essential for EXD nuclear localization and function. Mech Dev 91:279–291
Jia J, Amanai K, Wang G, Tang J, Wang B, Jiang J (2002) Shaggy/GSK3 antagonizes hedgehog signalling by regulating Cubitus interruptus. Nature 416:548–552
Kaphingst K, Kunes S (1994) Pattern formation in the visual centers of the Drosophila brain: wingless acts via decapentaplegic to specify the dorsoventral axis. Cell 78:437–448
Kumar JP, Moses K (2001) The EGF receptor and Notch signaling pathways control the initiation of the morphogenetic furrow during Drosophila eye development. Development 128:2689–2697
Labhart T, Meyer EP (1999) Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech 47:368–379
Lee JD, Treisman JE (2001) The role of Wingless signaling in establishing the anteroposterior and dorsoventral axes of the eye disc. Development 128:1519–1529
Lee JD, Treisman JE (2002) Regulators of the morphogenetic furrow. In: Moses K (ed) Drosophila eye development results and problems in cell differentiation 37. Springer-Verlag, Berlin Heidelberg New York, pp 21–34
Lin HV, Rogulja A, Cadigan KM (2004) Wingless eliminates ommatidia from the edge of the developing eye through activation of apoptosis. Development 131:2409–2418
Ma C, Moses K (1995) Wingless and patched are negative regulators of the morphogenetic furrow and can affect tissue polarity in the developing Drosophila compound eye. Development 121:2279–2289
Mardon G, Solomon NM, Rubin GM (1994) Dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120:3473–3486
Maurel-Zaffran C, Treisman JE (2000) Pannier acts upstream of wingless to direct dorsal eye disc development in Drosophila. Development 127:1007–1016
McNeill H, Yang CH, Brodsky M, Ungos J, Simon MA (1997) Mirror encodes a novel PBX-class homeoprotein that functions in the definition of the dorsal-ventral border in the Drosophila eye. Genes Dev 11:1073–1082
Meinertzhagen IA, Hanson TH (1993) The development of the optic lobe. In: Bate M, Martinez-Arias A (eds) The development of Drosophila melanogaster, vol. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y., pp 1363–1491
Myers PZ, Bastiani MJ (1993) Growth cone dynamics during the migration of an identified commissural growth cone. J Neurosci 13:127–143
Nagy LM, Carroll S (1994) Conservation of wingless patterning functions in the short-germ embryos of Tribolium castaneum. Nature 367:460–463
Niwa N, Inoue Y, Nozawa A, Saito M, Misumi Y, Ohuchi H, Yoshioka H, Noji S (2000) Correlation of diversity of leg morphology in Gryllus bimaculatus (cricket) with divergence in dpp expression pattern during leg development. Development 127:4373–4381
Nulsen C, Nagy LM (1999) The role of wingless in the development of multibranched crustacean limbs. Dev Genes Evol 209:340–348
Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801
Oppenheimer DI, MacNicol AM, Patel NH (1999) Functional conservation of the wingless-engrailed interaction as shown by a widely applicable baculovirus misexpression system. Curr Biol 9:1288–1296
Orsulic S, Peifer M (1994) A method to stain nuclei of Drosophila for confocal microscopy. Biotechniques 16:441–447
Pai CY, Kuo TS, Jaw, TJ, Kurant E, Chen CT, Bessarab DA, Salzberg A, Sun YH (1998) The Homothorax homeoprotein activates the nuclear localization of another homeoprotein, Extradenticle, and suppresses eye development in Drosophila. Genes Dev 12:435–446
Panin VM, Papayannopoulos V, Wilson R, Irvine KD (1997) Fringe modulates Notch-ligand interactions. Nature 387:908–912
Papayannopoulos V, Tomlinson A, Panin VM, Rauskolb C, Irvine KD (1998) Dorsal-ventral signaling in the Drosophila eye. Science 281:2031–2034
Pappu K, Mardon G (2002) Retinal specification and determination in Drosophila. In: Moses K (ed) Drosophila eye development results and problems in cell differentiation 37. Springer-Verlag, Berlin Heidelberg New York, pp 5–19
Pichaud F, Casares F (2000) Homothorax and iroquois-C genes are required for the establishment of territories within the developing eye disc. Mech Dev 96:15–25
Pignoni F, Zipursky SL (1997) Induction of Drosophila eye development by decapentaplegic. Development 124:271–278
Quiring R, Walldorf U, Kloter U, Gehring WJ (1994) Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265:785–789
Ramain P, Heitzler P, Haenlin M, Simpson P (1993) Pannier, a negative regulator of achaete and scute in Drosophila, encodes a zinc finger protein with homology to the vertebrate transcription factor GATA-1. Development 119:1277–1291
Ready DF, Hanson TE, Benzer S (1976) Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol 53:217–240
Reifegerste R, Moses K (1999) Genetics of epithelial polarity and pattern in the Drosophila retina. BioEssays 21:275–285
Richter S, Hartmann B, Reichert H (1998) The wingless gene is required for embryonic brain development in Drosophila. Dev Genes Evol 208:37–45
Rieckhof GE, Casares F, Ryoo HD, Abu-Shaar M, Mann RS (1997) Nuclear translocation of extradenticle requires homothorax, which encodes an extradenticle-related homeodomain protein. Cell 91:171–183
Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R (1987) The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell 50:649–657
Royet J, Finkelstein R (1996) Hedgehog, wingless and orthodenticle specify adult head development in Drosophila. Development 122:1849–1858
Royet J, Finkelstein R (1997) Establishing primordia in the Drosophila eye-antennal imaginal disc: the roles of decapentaplegic, wingless and hedgehog. Development 124:4793–4800
Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K (2002) The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417:295–299
Serikaku MA, O’Tousa JE (1994) Sine oculis is a homeobox gene required for Drosophila visual system development. Genetics 138:1137–1150
Singh A, Choi KW (2003) Initial state of the Drosophila eye before dorsoventral specification is equivalent to ventral. Development 130:6351–6360
Singh A, Kango-Singh M, Sun YH (2002) Eye suppression, a novel function of teashirt, requires Wingless signaling. Development 129:4271–4280
Slusarski DC, Yang-Snyder J, Busa WB, Moon RT (1997) Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev Biol 182:114–120
Stambolic V, Ruel L, Woodgett JR (1996) Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr Biol 6:1664–1668
Sucena E, Delon I, Jones I, Payre F, Stern DL (2003) Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature 424:935–938
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tomlinson A (2003) Patterning the peripheral retina of the fly: decoding a gradient. Dev Cell 5:799–809
Treisman JE, Rubin GM (1995) Wingless inhibits morphogenetic furrow movement in the Drosophila eye disc. Development 121:3519–3527
van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88:789–799
Wada S (1974) Spezielle randzonale Ommatidien der Fliegen (Diptera: Brachycera). Architektur und Verteilung in den Komplexaugen. Z Morphol Tiere 77:87–125
Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C (2003) Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell 115:267–279
Wiersdorff V, Lecuit T, Cohen SM, Mlodzik M (1996) Mad acts downstream of Dpp receptors, revealing a differential requirement for dpp signaling in initiation and propagation of morphogenesis in the Drosophila eye. Development 122:2153–2162
Wilson M, Garrard P, McGinness S (1978) The unit structure of the locust compound eye. Cell Tissue Res 195:205–226
Yang CH, Simon MA, McNeill H (1999) Mirror controls planar polarity and equator formation through repression of fringe expression and through control of cell affinities. Development 126:5857–5866
Younossi-Hartenstein Tepass U, Hartenstein V (1993) Embryonic origin of the imaginal discs of the head of Drosophila melanogaster. Roux’s Arch Dev Biol 203:60–73
Acknowledgements
We are indebted to Peter Dearden, Michael Akam and Robin White for providing probes and antibodies. This research was supported by NSF grants DBI-0070099 and DBI-0091926.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by C. Desplan
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Dong, Y., Friedrich, M. Comparative analysis of Wingless patterning in the embryonic grasshopper eye. Dev Genes Evol 215, 177–197 (2005). https://doi.org/10.1007/s00427-004-0465-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-004-0465-6