Abstract
Main conclusion
The present study is the first to integrate physiological and proteomic data providing information on Fe, Mn and Zn deficiency-responsive mechanisms of potato plants in vitro.
Abstract
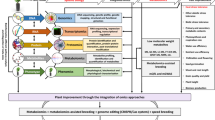
Micronutrient deficiency is an important limiting factor for potato production that causes substantial tuber yield and quality losses. To under the underlying molecular mechanisms of potato in response to Fe, Mn and Zn deficiency, a comparative proteomic approach was applied. Leaf proteome change of in vitro-propagated potato plantlets subjected to a range of Fe-deficiency treatments (20, 10 and 0 μM Na-Fe-EDTA), Mn-deficiency treatments (1 and 0 μM MnCl2·4H2O) and Zn-deficiency treatment (0 μM ZnCl2) using two-dimensional gel electrophoresis was analyzed. Quantitative image analysis showed a total of 146, 55 and 42 protein spots under Fe, Mn and Zn deficiency with their abundance significantly altered (P < 0.05) more than twofold, respectively. By MALDI-TOF/TOF MS analyses, the differentially abundant proteins were found mainly involved in bioenergy and metabolism, photosynthesis, defence, redox homeostasis and protein biosynthesis/degradation under the metal deficiencies. Signaling, transport, cellular structure and transcription-related proteins were also identified. The hierarchical clustering results revealed that these proteins were involved in a dynamic network in response to Fe, Mn and Zn deficiency. All these metal deficiencies caused cellular metabolic remodeling to improve metal acquisition and distribution in potato plants. The reduced photosynthetic efficiency occurred under each metal deficiency, yet Fe-deficient plants showed a more severe damage of photosynthesis. More defence mechanisms were induced by Fe deficiency than Mn and Zn deficiency, and the antioxidant systems showed different responses to each metal deficiency. Reprogramming of protein biosynthesis/degradation and assembly was more strongly required for acclimation to Fe deficiency. The signaling cascades involving auxin and NDPKs might also play roles in micronutrient stress signaling and pinpoint interesting candidates for future studies. Our results first provide an insight into the complex functional and regulatory networks in potato plants under Fe, Mn and Zn deficiency.









Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- 2-DE:
-
Two-dimensional gel electrophoresis
- DHAR:
-
Dehydroascorbate reductase
- Fd:
-
Ferredoxin
- FDH:
-
Formate dehydrogenase
- FeSOD:
-
Superoxide dismutase [Fe]
- FNR:
-
Ferredoxin NADP+ reductase
- NDPKs:
-
Nucleoside diphosphate kinases
- nsLTP:
-
Non-specific lipid-transfer protein
- OEE:
-
Oxygen-evolving enhancer protein
References
Abadía J, Vázquez S, Rellán-Álvarez R, El-Jendoubi H, Abadía A, Alvarez-Fernández A, López-Millán AF (2011) Towards a knowledge-based correction of iron chlorosis. Plant Physiol Biochem 49:471–482
Alekseeva AA, Savin SS, Tishkov VI (2011) NAD(+)-dependent formate dehydrogenase from plants. Acta Nat 3:38–54
Aliverti A, Pandini V, Pennati A, de Rosa M, Zanetti G (2008) Structural and functional diversity of ferredoxin-NADP+ reductases. Arch Biochem Biophys 474:283–291
Allen MD, Kropat J, Tottey S, Campo JAD, Merchant SS (2007) Manganese deficiency in Chlamydomonas results in loss of photosystem II and MnSOD function, sensitivity to peroxides, and secondary phosphorus and iron deficiency. Plant Physiol 143:263–277
Andreini C, Bertini I, Cavallaro G, Holliday G, Thornton J (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218
Assunção AG, Schat H, Aarts MG (2010a) Regulation of the adaptation to zinc deficiency in plants. Plant Signal Behav 5:1553–1555
Assunção AG, Herrero E, Lin YF, Huettel B, Talukdar S, Smaczniak C, Immink RG, van Eldik M, Fiers M, Schat H, Aarts MG (2010b) Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc Natl Acad Sci USA 107:10296–10301
Bacaicoa E, Zamarreño ÁM, Leménager D, Baigorri R, García-Mina JM (2009) Relationship between the hormonal balance and the regulation of iron deficiency stress responses in cucumber. J Am Soc Hortic Sci 134:589–601
Balk J, Schaedler TA (2014) Iron cofactor assembly in plants. Annu Rev Plant Biol 65:125–153
Bhushan D, Pandey A, Choudhary MK, Datta A, Chakraborty S, Chakraborty N (2007) Comparative proteomics analysis of differentially expressed proteins in chickpea extracellular matrix during dehydration stress. Mol Cell Proteomics 6:1868–1884
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20:33–40
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cailliatte R, Schikora A, Briat JF, Mari S, Curie C (2010) High affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell 22:904–917
Cakmak I (2000) Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Cakmak I, Pfeiffer WH, Mc Clafferty B (2010) Biofortification of durum wheat with zinc and iron. Cereal Chem 87:10–20
Carvalho AO, Gomes VM (2007) Role of plant lipid transfer proteins in plant cell physiology-a concise review. Peptides 28:1144–1153
Chatterjee C, Gopal R, Dube BK (2006) Impact of iron stress on biomass, yield, metabolism and quality of potato (Solanum tuberosum L.). Sci Hortic 108:1–6
Choudhary MK, Basu D, Datta A, Chakraborty N, Chakraborty S (2009) Dehydration responsive nuclear proteome of rice (Oryza sativa L.) illustrates protein network, novel regulators of cellular adaptation, and evolutionary perspective. Mol Cell Proteomics 8:1579–1598
Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54:183–206
De Pinto V, Messina A, Lane DJR, Lawen A (2010) Voltage-dependent anion-selective channel (VDAC) in the plasma membrane. FEBS Lett 584:1793–1799
Dixon DP, Cummins L, Cole DJ, Edwards R (1998) Glutathione-mediated detoxification systems in plants. Curr Opin Plant Biol 1:258–266
Donnelly BE, Madden RD, Ayoubi P, Porter DR, Dillwith JW (2005) The wheat (Triticum aestivum L.) leaf proteome. Proteomics 5:1624–1633
Dzeja PP, Terzic A (2009) Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing. Int J Mol Sci 10:1729–1772
Effendi Y, Rietz S, Fischer U, Scherer GF (2011) The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes. Plant J 65:282–294
Fukuyama K (2004) Structure and function of plant-type ferredoxins. Photosynth Res 81:289–301
Gangadhar BH, Sajeesh K, Venkatesh J, Baskar V, Abhinandan K, Yu JW, Prasad R, Mishra RK (2016) Enhanced tolerance of transgenic potato plants over-expressing non-specific lipid transfer protein-1 (StnsLTP1) against multiple abiotic stresses. Front Plant Sci 7:1228
Hagen G (2015) Auxin signal transduction. Essays Biochem 58:1–12
Hänsch R, Mendel RR (2009) Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol 12:259–266
Hantzis LJ, Kroh GE, Jahn CE, Cantrell M, Peers G, Pilon M, Ravet K (2018) A program for iron economy during deficiency targets specific Fe proteins. Plant Physiol 176:596–610
Haque ME, Yoshida Y, Hasunuma K (2010) ROS resistance in Pisum sativum cv. Alaska: the involvement of nucleoside diphosphate kinase in oxidative stress responses via the regulation of antioxidants. Planta 232:367–382
Hasunuma K, Yabe N, Yoshida Y, Ogura Y, Hamada T (2003) Putative functions of nucleoside diphosphate kinase in plants and fungi. J Bioenerg Biomembr 35:57–65
Heide H, Kalisz HM, Follmann H (2004) The oxygen evolving enhancer protein 1 (OEE) of photosystem II in green algae exhibits thioredoxin activity. J Plant Physiol 161:139–149
Hsieh SI, Castruita M, Malasarn D, Urzica E, Erde J, Page MD, Yamasaki H, Casero D, Pellegrini M, Merchant SS, Loo JA (2013) The proteome of copper, iron, zinc, and manganese micronutrient deficiency in Chlamydomonas reinhardtii. Mol Cell Proteomics 12:65–86
Ivanov R, Brumbarova T, Bauer P (2012) Fitting into the harsh reality: regulation of iron-deficiency responses in dicotyledonous plants. Mol Plant 5:27–42
Katayama H, Nagasu T, Oda Y (2001) Improvement of in-gel digestion protocol for peptide mass fingerprinting by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 15:1416–1421
Keil M, Sánchezserrano J, Schell J, Willmitzer L (1990) Localization of elements important for the wound-inducible expression of a chimeric potato proteinase inhibitor II-CAT gene in transgenic tobacco plants. Plant Cell 2:61–70
Khandakar J, Haraguchi I, Yamaguchi K, Kitamura Y (2013) A small-scale proteomic approach reveals a survival strategy, including a reduction in alkaloid biosynthesis, in Hyoscyamus albus roots subjected to iron deficiency. Front Plant Sci 4:331
Kim YH, Kim MD, Choi YI, Park SC, Yun DJ, Noh EW, Lee HS, Kwak SS (2011) Transgenic poplar expressing Arabidopsis NDPK2 enhances growth as well as oxidative stress tolerance. Plant Biotechnol J 9:334–347
Laganowsky A, Gómez SM, Whitelegge JP, Nishio JN (2009) Hydroponics on a chip: analysis of the Fe deficient Arabidopsis thylakoid membrane proteome. J Proteomics 72:397–415
Lan P, Li W, Wen TN, Shiau JY, Wu YC, Lin W, Schmidt W (2011) iTRAQ protein profile analysis of Arabidopsis roots reveals new aspects critical for iron homeostasis. Plant Physiol 155:821–834
Legay S, Guignard C, Ziebel J, Evers D (2012) Iron uptake and homeostasis related genes in potato cultivated in vitro under iron deficiency and overload. Plant Physiol Biochem 60:180–189
López-Millán AF, Morales F, Andaluz S, Gogorcena Y, Abadía A, De Las Rivas J, Abadía J (2000) Responses of sugar beet roots to iron deficiency. Changes in carbon assimilation and oxygen use. Plant Physiol 124:885–898
López-Millán AF, Grusak MA, Abadía A, Abadía J (2013) Iron deficiency in plants: an insight from proteomic approaches. Front Plant Sci 4:254
Ma F, Liu Z, Wang TW, Hopkins MT, Peterson CA, Thompson JE (2010) Arabidopsis eIF5A3 influences growth and the response to osmotic and nutrient stress. Plant Cell Environ 33:1682–1696
Marschner P (2012) Marschner’s mineral nutrition of higher plants. Academic Press, Boston
Matsuura-Endo C, Kobayashi A, Noda T, Takigawa S, Yamauchi H, Mori M (2004) Changes in sugar content and activity of vacuolar acid invertase during low-temperature storage of potato tubers from six Japanese cultivars. J Plant Res 117:131–137
Maurer F, Müller S, Bauer P (2011) Suppression of Fe deficiency gene expression by jasmonate. Plant Physiol Biochem 49:530–536
Millaleo R, Reyes-Díaz M, Ivanov AG, Mora ML, Alberdi M (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 10:476–494
Mills RF, Doherty ML, López-Marqués RL, Weimar T, Dupree P, Palmgren MG, Pittman JK, Williams LE (2008) ECA3, a Golgi-localized P2A-type ATPase, plays a crucial role in manganese nutrition in Arabidopsis. Plant Physiol 146:116–128
Milner MJ, Seamon J, Craft E, Kochian LV (2013) Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J Exp Bot 64:369–381
Morris J, Tian H, Park S, Sreevidya CS, Ward JM, Hirschi KD (2008) AtCCX3 is an Arabidopsis endomembrane H+-dependent K+ transporter. Plant Physiol 148:1474–1486
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nouet C, Motte P, Hanikenne M (2011) Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci 16:395–404
Paque S, Mouille G, Grandont L, Alabadí D, Gaertner C, Goyallon A, Muller P, Primard-Brisset C, Sormani R, Blázquez MA, Perrot-Rechenmann C (2014) AUXIN BINDING PROTEIN1 links cell wall remodeling, auxin signaling, and cell expansion in Arabidopsis. Plant Cell 26:280–295
Patron NJ, Rogers MB, Keeling PJ (2004) Gene replacement of fructose-1,6-bisphosphate aldolase supports the hypothesis of a single photosynthetic ancestor of chromalveolates. Eukaryot Cell 3:1169–1175
Pilon M, Cohu CM, Ravet K, Abdel-Ghany SE, Gaymard F (2009) Essential transition metal homeostasis in plants. Curr Opin Plant Biol 12:347–357
Potato Genome Sequencing Consortium (PGSC) (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195
Rellán-Álvarez R, Andaluz S, Rodríguez-Celma J, Wohlgemuth G, Zocchi G, Álvarez-Fernández A, Fiehn O, López-Millán AF, Abadía J (2010) Changes in the proteomic and metabolic profiles of Beta vulgaris root tips in response to iron deficiency and resupply. BMC Plant Biol 10:120
Rodnina M, Wintermeyer W (2011) The ribosome as a molecular machine: the mechanism of tRNA-mRNA movement in translocation. Biochem Soc Trans 39:658–662
Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30:1041–1051
Sarowar S, Kim YJ, Kim KD, Hwang BK, Ok SH, Shin JS (2009) Overexpression of lipid transfer protein (LTP) genes enhances resistance to plant pathogens and LTP functions in long-distance systemic signaling in tobacco. Plant Cell Rep 28:419–427
Schippers JHM, Mueller-Roeber B (2010) Ribosomal composition and control of leaf development. Plant Sci 179:307–315
Schmidt SB, Jensen PE, Husted S (2016) Manganese deficiency in plants: the impact on photosystem II. Trends Plant Sci 21:622–632
Sétif P, Fischer N, Lagoutte B, Bottin H, Rochaix JD (2002) The ferredoxin docking site of photosystem I. BBA-Bioenergetics 1555:204–209
Sharma S (2007) Adaptation of photosynthesis under iron deficiency in maize. Plant Physiol 164:1261–1267
Singh BN, Mishra RN, Agarwal PK, Goswami M, Nair S, Sopory SK, Reddy MK (2004) A pea chloroplast translation elongation factor that is regulated by abiotic factors. Biochem Biophys Res Commun 320:523–530
Singh AK, Kumar R, Pareek A, Sopory SK, Singla-Pareek SL (2012) Overexpression of rice CBS domain containing protein improves salinity, oxidative, and heavy metal tolerance in transgenic tobacco. Mol Biotechnol 52:205–216
Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17:616–627
Strommer J (2011) The plant ADH gene family. Plant J 66:128–142
Sun L, Ruppert M, Sheludko Y, Warzecha H, Zhao Y, Stöckigt J (2008) Purification, cloning, functional expression and characterization of perakine reductase: the first example from the AKR enzyme family, extending the alkaloidal network of the plant Rauvolfia. J Plant Mol Biol 67:455–467
Suzuki K, Itai R, Suzuki K, Nakanishi H, Nishizawa NK, Yoshimura E, Mori S (1998) Formatede hydrogenase, an enzyme of anaerobic metabolism, is induced by iron deficiency in barley roots. Plant Physiol 116:725–736
Tanaka S, Mochizuki N, Nagatani A (2002) Expression of the AtGH3a gene, an Arabidopsis homologue of the soybean GH3 gene, is regulated by phytochrome B. Plant Cell Physiol 43:281–289
van Leeuwe MA, Stefels J (2007) Photosynthetic responses in Phaeocystis antarctica towards varying light and iron conditions. Biogeochemistry 83:61–70
Vreugdenhil D (2007) Potato biology and biotechnology. Elsevier, Wageningen
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci 9:244–252
Wintz H, Fox T, Wu YY, Feng V, Chen W, Chang HS, Zhu T, Vulpe C (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278:47644–47653
Yadavalli V, Neelam S, Rao ASVC, Reddy AR, Subramanyam R (2012) Differential degradation of photosystem I subunits under iron deficiency in rice. J Plant Physiol 169:753–759
Yamada S, Komori T, Hashimoto A, Kuwata S, Imaseki H, Kubo T (2000) Differential expression of plastidic aldolase genes in Nicotiana plants under salt stress. Plant Sci 154:61–69
Yang KA, Moon HJ, Kim GT, Lim CJ, Hong JC, Lim CO, Yun DJ (2003) NDP kinase 2 regulates expression of antioxidant genes in Arabidopsis. Proc Jpn Acad Ser Biol 79:86–91
Yi X, McChargue M, Laborde S, Frankel LK, Bricker TM (2005) The manganese-stabilizing protein is required for photosystem II assembly/stability and photoautotrophy in higher plants. J Biol Chem 280:16170–16174
Zaharieva BT, Abadía J (2003) Iron deficiency enhances the levels of ascorbate, glutathione, and related enzymes in sugar beet roots. Protoplasma 221:269–275
Zhang XX, Liu SK, Takano T (2008) Overexpression of a mitochondrial ATP synthase small subunit gene (AtMtATP6) confers tolerance to several abiotic stresses in Saccharomyces cerevisiae and Arabidopsis thaliana. Biotechnol Lett 30:1289–1294
Acknowledgements
This work was supported by programs for National Key R&D Program of China (2017YFD0101905), National Natural Science Foundation of China (31471433, 31171477), Gansu High Educational Scientific Special Project (2018C-17), Gansu Provincial Key Laboratory of Aridland Crop Science (GSCS-2016-02).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2019_3163_MOESM1_ESM.tif
Supplemental Fig. S1 The primary 2-DE gel maps at least three biological replicates for the potato leaf proteome of control (124 μM Na-Fe-EDTA) and Fe-deficiency treatments (20, 10 and 0 μM Na-Fe-EDTA) in vitro under Fe deficiency (TIFF 47071 kb)
425_2019_3163_MOESM2_ESM.tif
Supplemental Fig. S2 The primary 2-DE gel maps at least three biological replicates for the potato leaf proteome of control (100 μM MnCl2·4H2O) and Mn-deficiency treatments (1 and 0 μM MnCl2·4H2O) in vitro under Mn deficiency (TIFF 34022 kb)
425_2019_3163_MOESM3_ESM.tif
Supplemental Fig. S3 The primary 2-DE gel maps at least three biological replicates for the potato leaf proteome of control (30 μM ZnCl2) and Zn-deficiency treatment (0 μM ZnCl2) in vitro under Zn deficiency (TIFF 21350 kb)
425_2019_3163_MOESM4_ESM.tif
Supplemental Fig. S4 The detailed information on differentially abundant proteins within each cluster in the clustering analysis under Fe deficiency. The four columns of hierarchical cluster tree represent control (124 μM Na-Fe-EDTA) and Fe-deficiency treatments (20, 10 and 0 μM Na-Fe-EDTA), respectively. Each rows represent individual proteins. The up- and down-regulation of proteins are indicated in red and green, respectively. The intensity of colors is increased when the expression differences increased, as shown in the bar at the top. The differentially abundant proteins were grouped into eight clusters under Fe deficiency. The detailed information on these proteins within each cluster is presented, including the protein identification number and protein name (TIFF 889 kb)
425_2019_3163_MOESM5_ESM.tif
Supplemental Fig. S5 The detailed information on differentially abundant proteins within each cluster in the clustering analysis under Mn deficiency. The three columns of hierarchical cluster tree represent control (100 μM MnCl2·4H2O) and Mn-deficiency treatments (1 and 0 μM MnCl2·4H2O), respectively. Each rows represent individual proteins. The up- and down-regulation of proteins are indicated in red and green, respectively. The intensity of colors is increased when the expression differences increased, as shown in the bar at the top. The differentially abundant proteins were grouped into six clusters under Mn deficiency. The detailed information on these proteins within each cluster is presented, including the protein identification number and protein name (TIFF 496 kb)
425_2019_3163_MOESM6_ESM.tif
Supplemental Fig. S6 The detailed information on differentially abundant proteins within each cluster in the clustering analysis under Zn deficiency. The two columns of hierarchical cluster tree represent control (30 μM ZnCl2) and Zn-deficiency treatment (0 μM ZnCl2), respectively. Each rows represent individual proteins. The up- and down-regulation of proteins are indicated in red and green, respectively. The intensity of colors is increased when the expression differences increased, as shown in the bar at the top. The differentially abundant proteins were grouped into two clusters under Zn deficiency. The detailed information on these proteins within each cluster is presented, including the protein identification number and protein name (TIFF 308 kb)
425_2019_3163_MOESM8_ESM.docx
Supplemental Table S2 Identification of differentially abundant proteins of potato leaves in vitro under Fe deficiency (DOCX 14218 kb)
425_2019_3163_MOESM9_ESM.docx
Supplemental Table S3 Identification of differentially abundant proteins of potato leaves in vitro under Mn deficiency (DOCX 198 kb)
425_2019_3163_MOESM10_ESM.docx
Supplemental Table S4 Identification of differentially abundant proteins of potato leaves in vitro under Zn deficiency (DOCX 141 kb)
425_2019_3163_MOESM11_ESM.xlsx
Supplemental Table S5 The primary identification information of differentially abundant proteins of potato leaves in vitro under Fe deficiency using MALDI-TOF/TOF MS. Protein identifications were performed by searching for the NCBInr-S. tuberosum database using peptide mass fingerprinting (PMF) and MS/MS data from a MALDI-TOF/TOF mass spectrometry analysis. a The spot number of identified proteins. b Gene identification number as in GenBank. c The number of matched peptides. d MASCOT score reported after searching against the NCBInr database. e The sequence coverage percentage (%). f Theoretical pI and mass (Da) values of identified proteins estimated with MASCOT (XLSX 194 kb)
425_2019_3163_MOESM12_ESM.xlsx
Supplemental Table S6 The primary identification information of differentially abundant proteins of potato leaves in vitro under Mn deficiency using MALDI-TOF/TOF MS. Protein identifications were performed by searching for the NCBInr-S. tuberosum database using peptide mass fingerprinting (PMF) and MS/MS data from a MALDI-TOF/TOF mass spectrometry analysis. a The spot number of identified proteins. b Gene identification number as in GenBank. c The number of matched peptides. d MASCOT score reported after searching against the NCBInr database. e The sequence coverage percentage (%). f Theoretical pI and mass (Da) values of identified proteins estimated with MASCOT (XLSX 88 kb)
425_2019_3163_MOESM13_ESM.xlsx
Supplemental Table S7 The primary identification information of differentially abundant proteins of potato leaves in vitro under Zn deficiency using MALDI-TOF/TOF MS. Protein identifications were performed by searching for the NCBInr-S. tuberosum database using peptide mass fingerprinting (PMF) and MS/MS data from a MALDI-TOF/TOF mass spectrometry analysis. a The spot number of identified proteins. b Gene identification number as in GenBank. c The number of matched peptides. d MASCOT score reported after searching against the NCBInr database. e The sequence coverage percentage (%). f Theoretical pI and mass (Da) values of identified proteins estimated with MASCOT (XLSX 70 kb)
Rights and permissions
About this article
Cite this article
Cheng, L., Zhang, S., Yang, L. et al. Comparative proteomics illustrates the complexity of Fe, Mn and Zn deficiency-responsive mechanisms of potato (Solanum tuberosum L.) plants in vitro. Planta 250, 199–217 (2019). https://doi.org/10.1007/s00425-019-03163-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-019-03163-w