Abstract
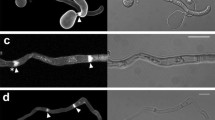
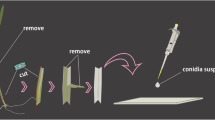
Induced penetration resistance is triggered by failed penetration attempts of nonpathogenic fungi. The resistance mechanism is an important nonhost reaction in plants that can block the invasion of filamentous pathogens such as fungi and oomycetes. However, it remains unclear whether the mechanical stimuli accompanying fungal penetration play a role in induced penetration resistance, whereas the perforation of the cell wall may provide significant stimuli to plant cells. Here, we used microneedles or biolistic bombardment to mimic fungal penetration pegs and a micromanipulation transfer technique of the bio-probe, a germling of Blumeria graminis hordei, to the wounded cells to demonstrate that microwounds derived from fungal penetration attempts may trigger induced penetration resistance in plant cells. When preinoculated with the nonpathogenic fungi Erysiphe pisi and Colletotrichum orbiculare, which were unable to penetrate a barley cell, the penetration of a bio-probe that was transferred by micromanipulation onto the same cell was completely blocked. Fungal penetration was essential to the triggering of induced penetration resistance because a penetration-peg-defective mutant of C. orbiculare completely lacked the ability to trigger resistance. The artificial microwounds significantly, but not completely, blocked the penetration of the bio-probe. Treatment with the actin polymerization inhibitor cytochalasin A or expression of the actin depolymerizing protein HvPro1 caused complete ablation of the induced penetration resistance triggered by either failed fungal penetration or artificial microwounds. These results strongly suggest that microwounding may trigger actin-dependent induced penetration resistance. Manipulation of induced penetration resistance may be a promising target to improve basic disease resistance in plants.





Similar content being viewed by others
Abbreviations
- DAMP:
-
Damage-associated molecular pattern
- DIC:
-
Differential interference contrast
- Loc :
-
Mutant with loss of cellulase secretion
- Lop :
-
Mutant with loss of penetration peg formation
- MAMP:
-
Microbe-associated molecular pattern
- PAMP:
-
Pathogen-associated molecular pattern
References
Aist JR (1976) Papillae and related wound plugs of plant cells. Annu Rev Phytopathol 14:145–163
Chen Z, Nunes MA, Silva MC, Rodrigues CJ Jr (2004) Appressorium turgor pressure of Colletotrichum kahawae might have a role in coffee cuticle penetration. Mycologia 96:1199–1208
Clement JA, Martin SG, Porter R, Butt TM, Beckett A (1994) The role of hydrophobicity in attachment of urediniospores and sporelings of Uromyces. Germination and the role of extracellular matrix in adhesion of urediniospores of Uromyces viciae-fabae. Mycol Res 98:1217–1228
Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16:524–531
Collins NC, Thordal-Christensen H, Lipka V, Bau S, Kombrink E, Qiu JL, Hückelhoven R, Stein M, Freialdenhoven A, Somerville SC, Schulze-Lefert P (2003) SNARE-protein-mediated disease resistance at the plant cell wall. Nature 425:973–977
Davis KR, Hahlbrock K (1987) Induction of defense responses in cultured parsley cells by plant cell wall fragments. Plant Physiol 84:1286–1290
Enkerli J, Felix G, Boller T (1999) The enzymatic activity of fungal xylanase is not necessary for its elicitor activity. Plant Physiol 121:391–397
Fauth M, Schweizer P, Buchala A, Markstädter C, Riederer M, Kato T, Kauss H (1998) Cutin monomers and surface wax constituents elicit H2O2 in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2 elicitors. Plant Physiol 117:1373–1380
Feng J, Wang F, Liu G, Greenshields D, Shen W, Kaminskyj S, Hughes GR, Peng Y, Selvaraj G, Zou J, Wei Y (2009) Analysis of a Blumeria graminis-secreted lipase reveals the importance of host epicuticular wax components for fungal adhesion and development. Mol Plant Microbe Interact 22:1601–1610
Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, De Lorenzo G, Ferrari S (2008) The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol 148:1695–1706
Gus-Mayer S, Naton B, Hahlbrock K, Schmelzer E (1998) Local mechanical stimulation induces components of the pathogen defense response in parsley. Proc Natl Acad Sci USA 95:8398–8403
Hardham AR, Jones DA, Takemoto D (2007) Cytoskeleton and cell wall function in penetration resistance. Curr Opin Plant Biol 10:342–348
Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM (2008) Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol 18:730–734
Howard RJ, Ferrari MA, Roach DH, Money NP (1991) Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc Natl Acad Sci USA 88:11281–11284
Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT (2009) Priming in systemic plant immunity. Science 324:89–91
Katoh M, Hirose I, Kubo Y, Hikichi Y, Kunoh H, Furusawa I, Shishiyama J (1988) Use of mutants to indicate factors prerequisite for penetration of Colletotrichum lagenarium by appressoria. Physiol Mol Plant Pathol 32:177–184
Kobayashi I, Komura T, Sakamoto Y, Yamaoka N, Kunoh H (1990) Recognition of a pathogen and a nonpathogen by barley coleoptile cells. (I) Cytoplasmic responses to the nonpathogen, Erysiphe pisi, prior to its penetration. Physiol Mol Plant Pathol 37:479–490
Kobayashi I, Watanabe H, Kunoh H (1995) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. XIV Evidence for elicitor(s) and suppressor(s) of host inaccessibility from Erysiphe graminis. Physiol Mol Plant Pathol 46:445–456
Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H (1997) Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. Plant J 11:525–537
Kobayashi I, Yamada M, Kobayashi Y (2007) Calcium ion promotes successful penetration of powdery mildew fungi into barley cells. J Gen Plant Pathol 73:399–404
Kováts K, Binder A, Hohl HR (1991) Cytology of induced systemic resistance of cucumber to Colletotrichum lagenarium. Planta 183:484–490
Kunoh H, Hayashimoto A, Harui M, Ishizaki H (1985) Induced accessibility and enhanced resistance at the cellular level in barley coleoptiles. I The significance of timing of fungal invasion. Physiol Plant Pathol 27:43–54
Kunoh H, Katsuragawa N, Yamaoka N, Hayashimoto A (1988) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. III Timing and localization of enhanced inaccessibility in a single coleoptile cell and its transfer to an adjacent cell. Physiol Mol Plant Pathol 33:81–93
Kunoh H, Toyoda K, Yamaoka N, Kobayashi I (1989) Induced accessibility and enhanced inaccessibility at the cellular level in barley coleoptiles. V Duration of stimulus by a non-pathogen in relation to enhanced inaccessibility. Physiol Mol Plant Pathol 35:507–518
Lipka V, Dittgen J, Bednarek P, Bhat R, Wiermer M, Stein M, Landtag J, Brandt W, Rosahl S, Scheel D, Llorente F, Molina A, Parker J, Somerville S, Schulze-Lefert P (2005) Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310:1180–1183
Lu JP, Liu XH, Feng XX, Min H, Lin FC (2009) An autophagy gene, MgATG5, is required for cell differentiation and pathogenesis in Magnaporthe oryzae. Curr Genet 55:461–473
Matern U, Grimmig B, Kneusel RE (1995) Plant cell wall reinforcement in the disease resistance response—molecular composition and regulation. Can J Bot 73:S511–S517
Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, Kato T, Tabata S, Iida K, Terashima A, Nakano M, Ikeda M, Yamanaka T, Iida H (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104:3639–3644
Nguyen QB, Itoh K, Van Vu B, Tosa Y, Nakayashiki H (2011) Simultaneous silencing of endo-β-1,4 xylanase genes reveals their roles in the virulence of Magnaporthe oryzae. Mol Microbiol 81:1008–1019
Nielsen KA, Nicholson RL, Carver TLW, Kunoh H, Oliver RP (2000) First touch: an immediate response to surface recognition in conidia of Blumeria graminis. Physiol Mol Plant Pathol 56:63–70
Niwa Y, Hirano T, Yoshimoto K, Shimizu M, Kobayashi H (1999) Non-invasive quantitative detection and applications of non-toxic S65T-type green fluorescent protein in living plants. Plant J 18:455–463
Park G, Bruno KS, Staiger CJ, Talbot NJ, Xu JR (2004) Independent genetic mechanisms mediate turgor generation and penetration peg formation during plant infection in the rice blast fungus. Mol Microbiol 53:1695–1707
Perpetua NS, Kubo Y, Okuno T, Furusawa I (1994) Restoration of pathogenicity of a penetration-deficient mutant of Collectotrichum lagenarium by DNA complementation. Curr Genet 25:41–46
Prime-A-Plant Group, Conrath U, Beckers GJ, Flors V, García-Agustín P, Jakab G, Mauch F, Newman MA, Pieterse CM, Poinssot B, Pozo MJ, Pugin A, Schaffrath U, Ton J, Wendehenne D, Zimmerli L, Mauch-Mani B (2006) Priming: getting ready for battle. Mol Plant Microbe Interact 19:1062–1071
Schulze-Lefert P (2004) Knocking on the heaven’s wall: pathogenesis of and resistance to biotrophic fungi at the cell wall. Curr Opin Plant Biol 7:377–383
Shimada C, Lipka V, O’Connell R, Okuno T, Schulze-Lefert P, Takano Y (2006) Nonhost resistance in Arabidopsis–Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol Plant Microbe Interact 19:270–279
Shimono M, Sugano S, Nakayama A, Jiang CJ, Ono K, Toki S, Takatsuji H (2007) Rice WRKY45 plays a crucial role in benzothiadiazole-inducible blast resistance. Plant Cell 19:2064–2076
Shimono M, Koga H, Akagi A, Hayashi N, Goto S, Sawada M, Kurihara T, Matsushita A, Sugano S, Jiang CJ, Kaku H, Inoue H, Takatsuji H (2012) Rice WRKY45 plays important roles in fungal and bacterial disease resistance. Mol Plant Pathol 13:83–94
Skamnioti P, Gurr SJ (2007) Magnaporthe grisea cutinase2 mediates appressorium differentiation and host penetration and is required for full virulence. Plant Cell 19:2674–2689
Staiger CJ, Yuan M, Valenta R, Shaw PJ, Warn RM, Lloyd CW (1994) Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr Biol 4:215–219
Stein M, Dittgen J, Sánchez-Rodríguez C, Hou BH, Molina A, Schulze-Lefert P, Lipka V, Somerville S (2006) Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 18:731–746
Stumm D, Gessler C (1986) Role of papillae in the induced systemic resistance of cucumber against Colletotrichum lagenarium. Physiol Mol Plant Pathol 29:405–410
Takemoto D, Jones DA, Hardham AR (2003) GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33:775–792
Tominaga M, Yokota E, Vidali L, Sonobe S, Hepler PK, Shimmen T (2000) The role of plant villin in the organization of the actin cytoskeleton, cytoplasmic streaming and the architecture of the transvacuolar strand in root hair cells of Hydrocharis. Planta 210:836–843
Tonukari NJ, Scott-Craig JS, Walton JD (2000) The Cochliobolus carbonum SNF1 gene is required for cell wall-degrading enzyme expression and virulence on maize. Plant Cell 12:237–248
Toyoda K, Kobayashi I, Kunoh H (1993) Elicitor activity of a fungal product assessed at the single-cell level by a novel gel-bead method. Plant Cell Physiol 34:775–780
Tsuji G, Fujii S, Tsuge S, Shiraishi T, Kubo Y (2003) The Colletotrichum lagenarium Ste12-like gene CST1 is essential for appressorium penetration. Mol Plant Microbe Interact 16:315–325
Underwood W, Somerville SC (2008) Focal accumulation of defences at sites of fungal pathogen attack. J Exp Bot 59:3501–3508
Xu JR, Staiger CJ, Hamer JE (1998) Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc Natl Acad Sci USA 95:12713–12718
Zhang Z, Henderson C, Perfect E, Carver TLW, Thomas BJ, Skamnioti P, Gurr SJ (2005) Of genes and genomes, needles and haystacks: Blumeria graminis and functionality. Mol Plant Pathol 6:561–575
Acknowledgments
We are grateful to Dr. Yasuyuki Kubo (Laboratory of Plant Pathology, Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Kyoto, Japan) for the gift of Colletotrichum orbiculare mutants. This work was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Integrated research project for plant, insect and animal using genome technology PMI0006 and Grant-in-Aid for Scientific Research No. 23580062 (2011–2013) from the Ministry of Education, Science and Culture of Japan, both given to I. Kobayashi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, Y., Kobayashi, I. Microwounding is a pivotal factor for the induction of actin-dependent penetration resistance against fungal attack. Planta 237, 1187–1198 (2013). https://doi.org/10.1007/s00425-013-1837-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-013-1837-9