Abstract
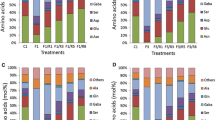
The effects of dark-induced stress on the evolution of the soluble metabolites present in senescent soybean (Glycine max L.) nodules were analysed in vitro using 13C- and 31P-NMR spectroscopy. Sucrose and trehalose were the predominant soluble storage carbons. During dark-induced stress, a decline in sugars and some key glycolytic metabolites was observed. Whereas 84% of the sucrose disappeared, only one-half of the trehalose was utilised. This decline coincides with the depletion of Gln, Asn, Ala and with an accumulation of ureides, which reflect a huge reduction of the N2 fixation. Concomitantly, phosphodiesters and compounds like P-choline, a good marker of membrane phospholipids hydrolysis and cell autophagy, accumulated in the nodules. An autophagic process was confirmed by the decrease in cell fatty acid content. In addition, a slight increase in unsaturated fatty acids (oleic and linoleic acids) was observed, probably as a response to peroxidation reactions. Electron microscopy analysis revealed that, despite membranes dismantling, most of the bacteroids seem to be structurally intact. Taken together, our results show that the carbohydrate starvation induced in soybean by dark stress triggers a profound metabolic and structural rearrangement in the infected cells of soybean nodule which is representative of symbiotic cessation.



Similar content being viewed by others
Abbreviations
- Fru6P:
-
Fructose 6-phosphate
- GABA:
-
γ-Aminobutyric acid
- Glc6P:
-
Glucose 6-phosphate
- Glyc3P:
-
Glycerol 3-phosphate
- GPC:
-
Glycerylphosphoryl-choline
- GPE:
-
Glycerylphosphoryl-ethanolamine
- GPG:
-
Glycerylphosphoryl-glycerol
- GPI:
-
Glycerylphosphoryl-inositol
- Man6P:
-
Mannose 6-phosphate
- PBM:
-
Peribacteroid membrane
- P-cho:
-
Phosphoryl-choline
- P-eth:
-
Phosphoryl-ethanolamine
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
References
Andreeva IN, Kozharinova GM, Izmailov SF (1998) Senescence of legume nodules. Russ J Plant Physiol 45:101–112
Anthon GE, Emerich DW (1990) Developmental regulation of enzymes of sucrose and hexose metabolism in effective and ineffective soybean nodules. Plant Physiol 92:346–351
Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R (1996) Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by supply of mitochondria with respiratory substrates. J Cell Biol 133:1251–1263
Bassarab S, Schenk SU, Werner D (1989) Fatty acid composition of the peribacteroid membrane and the ER in nodules of Glycine max varies after infection by different strains of the microsymbiont Bradyrhizobium japonicum. Bot Acta 102:196–201
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bouché N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Broughton WJ (2003) Roses by other names: taxonomy of the Rhizobiaceae. J Bacteriol 185:2975–2979
Ching TM, Hedtke S, Russel SA, Evans HJ (1975) Energy state and dinitrogen fixation in soybean nodules of dark-grown plants. Plant Physiol 55:796–798
Cohen HP, Sarath G, Lee K, Wagner FW (1986) Soybean root nodule ultrastructure during dark-induced stress and recovery. Protoplasma 132:69–75
Cots J, Fargeix C, Gindro K, Widmer F (2002) Pathogenic attack and carbon reallocation in soybean leaves (Glycine max L.): reinitiation of the glycoxylate cycle as a defense reaction. J Plant Physiol 159:91–96
Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83:3811–3815
Day DA, Copeland L (1991) Carbon metabolism and compartmentation in nitrogen-fixing legume nodules. Plant Physiol Biochem 29:185–201
Day DA, Price GD, Gresshof PM (1987) A comparison of mitochondria from soybean nodules, roots and cotyledons. In: Moore AL, Beechey RB (eds) Plant mitochondria: structural, functional and physiological aspects. Plenum Press, New York, pp 207–210
Day DA, Poole PS, Tyerman SD, Rosendahl L (2001) Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell Mol Life Sci 58:61–71
Dorne A-J, Bligny R, Rébeillé F, Roby C, Douce R (1987) Fatty acid disappearance and phosphorylcholine accumulation in higher plant cells after a long period of sucrose deprivation. Plant Physiol Biochem 25:589–595
Doyle JJ, Luckow MA (2003) The rest of the iceberg Legume diversity and evolution in a phylogenetic context. Plant Physiol 131:900–910
Elbein AD, Pan YT, Pastuszak I, Carroll D (2003) New insights on trehalose: a multifunctional molecule. Glycobiology 13(4):17R–27R
Espinosa-Victoria D, Vance CP, Graham PH (2000) Host variation in traits associated with crown nodule senescence in soybean. Crop Sci 40:103–109
Fargeix C, Gindro K, Widmer F (2004) Soybean (Glycine max L.) and bacteroid glyoxylate cycle activities during nodular senescence. J Plant Physiol 161:183–190
Galliard T (1980) Degradation of acyl lipids: hydrolytic and oxidative enzymes. In: Stumpf PK (ed) The biochemistry of plants. A comprehensive treatise, vol 4. Academic Press, New York, pp 85–116
Gordon AJ, Ougham HJ, James CL (1993) Changes in the levels of gene transcripts and their corresponding proteins in nodules of soybean plant subjected to dark-induced stress. J Exp Bot 44:1453–1460
Gout E, Aubert S, Bligny R, Rébeillé F, Nonomura AR, Benson AA, Douce R (2000) Metabolism of methanol in plant cells. Carbon-13 nuclear magnetic resonance studies. Plant Physiol 123:287–296
Hong ZQ, Copeland L (1990) Pentose phosphate pathway enzymes in nitrogen-fixing leguminous root nodules. Phytochemistry 29:2437–2440
Jacobi A, Katinakis P, Werner D (1994) Artificially induced senescence of soybean root nodules affects different polypeptides and nodulins in the symbiosome membrane compared to physiological ageing. J Plant Physiol 144:533–540
King CA, Purcell LC (2005) Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol 137:1389–1396
Kuzma MM, Winter H, Storer P, Oresnik II, Atkins CA, Layzell DB (1999) The site of oxygen limitation in soybean nodules. Plant Physiol 119:399–408
Lee JW, Zhang Y, Weaver CD, Shomer NH, Louis CF, Roberts DM (1995) Phosphorylation of nodulin 26 on serine 262 affects its voltage-sensitive channel activity in planar lipid bilayers. J Biol Chem 270:27051–27057
Lodwig E, Poole P (2003) Metabolism of Rhizobium bacteroids. Crit Rev Plant Sci 22:37–78
Lord JM (1987) Isolation of endoplasmic reticulum: general principles, enzymatic markers, and endoplasmic reticulum bound polysomes. Methods Enzymol 148:576–584
Matamoros MA, Baird LM, Escurado PR, Dalton DA, Minchin FR, Iturbe-Ormaetxe I, Rubio MC, Moran JF, Gordon AJ, Becana M (1999) Stress-induced legume root nodule senescence physiological, biochemical, and structural alteration. Plant Physiol 121:97–111
Matamoros MA, Loscos J, Coronado MJ, Ramos J, Sato S, Testillano PS, Tabata S, Becana M (2006) Biosynthesis of ascorbic acid in legume root nodules. Plant Physiol 141:1068–1077
Miao GH, Verma DP (1993) Soybean nodulin-26 gene encoding a channel protein is expressed only in the infected cells of nodules and is regulated differently in roots of homologous and heterologous plants. Plant Cell 5:781–794
Müller J, Xie Z-P, Staehelin C, Mellor RB, Boller T, Wiemken A (1994) Trehalose and trehalase in root nodules from various legumes. Physiol Plant 90:86–92
Müller J, Boller T, Wiemken A (1998) Trehalose affects sucrose synthase and invertase activities in soybean (Glycine max [L.] Merr.) roots. J Plant Physiol 153:255–257
Newton WE (2000) Nitrogen fixation. In: Pedrosa FO, Hungria M, Yates MG, Newton WE (eds) From molecules to crop productivity. Kluwer, Dordrecht, pp 3–8
Patriarca EJ, Tate R, Ferraioli S, Laccarino M (2004) Organogenesis of legume root nodules. Int Rev Cytol 234:201–262
Pau AS, Cowles JR (1979) Effect of induced nodule senescence on parameters related to dinitrogen fixation, bacteroid size, and nucleic acid content. J Gen Microbiol 111:101–107
Perret X, Staehelin C, Broughton WJ (2000) Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev 64:180–201
Pfeiffer NE, Malik NSA, Wagner FW (1983) Reversible dark-induced senescence of soybean root nodules. Plant Physiol 71:393–399
Prell J, Poole P (2006) Metabolic changes of rhizobia in legume nodules. Trends Microbiol 14:1–8
Puppo A, Herrada G, Rigaud J (1991) Lipid peroxidation in peribacteroid membrane from French bean nodules. Plant Physiol 96:826–830
Puppo A, Groten K, Bastian F, Carzaniga R, Soussi M, Mercedes Lucas M, Rosaria de Felipe M, Harisson J, Vanacker H, Foyer CH (2005) Legume nodule senescence: roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol 165:683–701
Quail PH (1979) Plant cell fractionation. Annu Rev Plant Physiol 30:425–484
Roby C, Martin JB, Bligny R, Douce R (1987) Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem 262:5000–5007
Rolland F, Baeana-Gonzalez E, Sheen J (2006) Sugar sensing and signalling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57:675–709
Schubert KR (1986) Products of biological nitrogen fixation in higher plants; synthesis, transport and metabolism. Annu Rev Plant Physiol 37:539–574
Streeter JG (1982) Enzymes of sucrose, maltose and alpha, alpha-trehalose catabolism in soybean root nodules. Planta 155:112–115
Streeter JG (1987) Carbohydrate, organic acid, and amino acid composition of bacteroids and cytosol from soybean nodules. Plant Physiol 85:768–773
Tajima S, Nomura M, Kouchi H (2004) Ureide biosynthesis in legume nodules. Front Biosci 9:1374–1381
Udvardi MK, Day DA (1997) Metabolite transport across symbiotic membranes of legume nodules. Annu Rev Plant Physiol Plant Mol Biol 48:493–523
Vadez V, Sinclair TR (2000) Ureide degradation pathways in intact soybean leaves. J Exp Bot 51:1459–1465
Vadez V, Sinclair TR (2001) Leaf ureide degradation and N2 fixation tolerance to water deficit in soybean. J Exp Bot 52:153–159
Van de Velde W, Pérez Guerra JC, De Keyser A, De Rycke R, Rombauts S, Maunoury N, Mergaert P, Kondorosi E, Holsters M, Goormachtig S (2006) Aging in legume symbiosis A molecular view on nodule senescence in Medicago truncatula. Plant Physiol 141:711–720
Van der Rest B, Boisson A-M, Gout E, Bligny R, Douce R (2002) Glycerophosphocholine metabolism in higher plant cells evidence of a new glyceryl-phosphodiester phosphodiesterase. Plant Physiol 130:244–255
Vauclare P, Cots J, Gindro K, Widmer F (2003) The glyoxylate cycle as an essential step in carbon reallocation mechanisms. Adv Plant Physiol 5:97–132
Weaver CD, Crombie B, Stacey G, Roberts DM (1991) Calcium-dependent phosphorylation of symbiosome membrane proteins from nitrogen-fixing soybean nodules: evidence for phosphorylation of Nodulin-26. Plant Physiol 95:222–227
Werner D, Mellor RB, Hahn MG, Grisebach H (1985) Soybean root response to symbiotic infection Glyceollin I accumulation in an ineffective type of soybean nodules with an early loss of the peribacteroid membrane. Z Naturfosch 40:179–181
Wheeler GL, Jones MA, Smirnoff N (1998) The biosynthetic pathway of vitamin C in higher plants. Nature 393:365–369
Zrenner R, Stitt M, Sonnewald U, Boldt R (2006) Pyrimidine and purine biosynthesis and degradation in plants. Annu Rev Plant Biol 57:805–836
Acknowledgments
We are grateful to Dr. John Lomas (ITODYS UMR 7086, Université Paris-Diderot, Paris 7) for his fine work in correction of English text in the manuscript. We also thank Josiane Bonetti for her excellent bibliographic assistance. This work was supported by grants from the University of Lausanne (Switzerland).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vauclare, P., Bligny, R., Gout, E. et al. Metabolic and structural rearrangement during dark-induced autophagy in soybean (Glycine max L.) nodules: an electron microscopy and 31P and 13C nuclear magnetic resonance study. Planta 231, 1495–1504 (2010). https://doi.org/10.1007/s00425-010-1148-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1148-3