Abstract
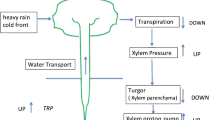
A steady supply of water is indispensable for leaves to fulfil their photosynthetic function. Understanding water movement in leaves, especially factors that regulate the movement of water flux from xylem to epidermis, requires that the nature of the transport pathway be elucidated. To determine the hydraulic linkage between xylem and epidermis, epidermal cell turgor pressure (P t) in leaves of Tradescantia fluminensis was monitored using a cell pressure probe in response to a 0.2 MPa step change in xylem pressure applied at the leaf petiole. Halftime of P t changes \( {\left( {T^{x}_{{1/2}} } \right)} \) were 10–30 times greater than that of water exchange across an individual cell membrane \( {\left( {T^{m}_{{1/2}} } \right)}, \) suggesting that cell-to-cell water transport constitutes a significant part of the leaf hydraulic path from xylem to epidermis. Furthermore, perfusion of H2O2 resulted in increases of both \( T^{m}_{{1/2}} \) and \( T^{x}_{{1/2}} \) by a factor of 2.5, indicating that aquaporins may play a role in the xylem to epidermis hydraulic link. The halftime for water exchange \( {\left( {T^{m}_{{1/2}} } \right)} \) did not differ significantly between cells located at the leaf base (2.5 s), middle (2.6 s) and tip (2.5 s), indicating that epidermal cell hydraulic properties are similar along the length of the leaf. Following the pressure application to the xylem (0.2 MPa), P t changed by 0.12, 0.06 and 0.04 MPa for epidermal cells at the base, middle and the tip of the leaf, respectively. This suggests that pressure dissipation between xylem and epidermis is significant, and that the pressure drop along the vein may be due to its structural similarities to a porous pipe, an idea which was further supported by measurements of xylem hydraulic resistance using a perfusion technique.







Similar content being viewed by others
References
Aroca R, Amodeo G, Fernandez-Illescas S, Herman EM, Chaumont F, Chrispeels MJ (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 137:341–353
Boyer JS (1974) Water transport in plants: mechanism of apparent changes in resistance during absorption. Planta 117:187–207
Boyer JS (1977) Regulation of water movement in whole plants. In: Jennings DH (ed) Integration of activity in the higher plant. Cambridge University Press, Cambridge, pp 455–470
Boyer JS (1985) Water transport. Annu Rev Plant Physiol 36:473–516
Canny MJ (1993) The transpiration stream in the leaf apoplast: water and solutes. Philos Trans R Soc Lond B 341:87–100
Chaumont F, Moshelion M, Daniels MJ (2005) Regulation of plant aquaporin activity. Biol Cell 97:749–764
Cochard H, Nardini A, Coll L (2004) Hydraulic architecture of leaf blades: where is the main resistance? Plant Cell Environ 27:1257–1267
Comstock JP, Mencuccini M (1998) Control of stomatal conductance by leaf water potential in Hymenoclea salsola (T&G.), a desert subshrub. Plant Cell Environ 21:1029–1038
Cosgrove D, Steudle E (1981) Water relations in growing pea epicotyl segments. Planta 153:343–350
Cruiziat P, Tyree M, Bodet C, Logullo M (1980) Kinetics of rehydration of detached sunflower leaves following substantial water loss. New Phytol 84:293–306
Fricke W (2000) Water movement between epidermal cells of barley leaves—a symplastic connection? Plant Cell Environ 23:991–997
Grams TEE, Koziolek C, Lautner S, Matyssek R, Fromm J (2007) Distinct roles of electric and hydraulic signals on the reaction of leaf gas exchange upon re-irrigation in Zea mays L. Plant Cell Environ 30:79–84
Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot 90:301–313
Kim YMX, Steudle E (2007) Light and turgor affect the water permeability (aquaporins) of parenchyma cells in the midrib of leaves of Zea mays. J Exp Bot 58:4119–4129
Kjellbom P, Larsson C, Johannson I, Karlsson M, Johansson U (1999) Aquaporins and water homeostasis in plants. Trends Plant Sci 4:308–314
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Landsberg JJ, Fowkes ND (1978) Water movement through plant roots. Ann Bot 42:493–508
Martre P, Morillon R, Barrieu F, North GB, Nobel PS, Chrispeels MJ (2002) Plasma membrane aquaporins play a significant role during recovery from water deficit. Plant Physiol 130:2101–2110
Maurel C (2007) Plant aquaporins: novel functions and regulation properties. FEBS Lett 581:2227–2236
Mott KA, Buckley TN (2000) Patchy stomatal conductance: emergent collective behaviour of stomata. Trends Plant Sci 5:258–262
Murphy R, Smith A (1998) Determination of cell water-relation parameters using the pressure probe: extended theory and practice of the pressure-clamp technique. Plant Cell Environ 21:637–657
Oparka K, Prior D (1992) Direct evidence for pressure-generated closure of plasmodesmata. Plant J 2:741–750
Pickard WF (1981) How does the shape of the substomatal chamber affect transpirational water loss? Math Biosci 56:111–127
Sack L, Holbrook NM (2006) Leaf hydraulics. Annu Rev Plant Biol 57:361–381
Sack L, Cowan P, Jaikumar N, Holbrook N (2003) The ‘hydrology’ of leaves: coordination of structure and function in temperate woody species. Plant Cell Environ 26:1343–1356
Sack L, Streeter CM, Holbrook NM (2004) Hydraulic analysis of water flow through leaves of sugar maple and red oak. Plant Physiol 134:1824–1833
Salleo S, Raimondo F, Trifilo P, Nardini A (2003) Axial-to-radial water permeability of leaf major veins: a possible determinant of the impact of vein embolism on leaf hydraulics? Plant Cell Environ 26:1749–1758
Sharkey T, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52:407–436
Steudle E (1993) Pressure probe techniques: basic principles and application to studies of water and solute relations at the cell, tissue, and organ level. In: Smith J, Griffith H (eds) Water deficits: plant responses from cell to community. BIOS Sicentific Publishers, Oxford, pp 5–36
Steudle E, Henzler T (1995) Water channels in plants: do basic concepts of water transport change. J Exp Bot 46:1067–1076
Steudle E, Jeschke WD (1983) Water transport in barley roots. Planta 158:237–248
Tomos AD, Steudle E, Zimmermann U, Schulze ED (1981) Water relations of leaf empidermal cells of Tradescantia virginiana. Plant Physiol 68:1135–1143
Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JA (1999) Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. J Exp Bot 25:1055–1071
Tyree M, Cruiziat P, Benis M, Logullo M, Salleo S (1981) The kinetics of rehydration of detached sunflower leaves from different initial water deficits. Plant Cell Environ 4:309–317
Tyree MT, Sobrado MA, Stratton LJ, Becker P (1999) Diversity of hydraulic conductance in leaves of temperate and tropical species: possible causes and consequences. J Trop For Sci 11:47–60
Tyree MT, Nardini A, Salleo S, Sack L, El Omari B (2005) The dependence of leaf hydraulic conductance on irradiance: any role for stomatal response? J Exp Bot 56:737–744
Volkov V, C Hachez, Moshelion M, Draye X, Chaumont F, Fricke W (2007) Water permeability differs between growing and non-growing barley leaf tissues. J Exp Bot 58:377–390
Wan X, Steudle E, Hartung W (2004) Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and HgCl2. J Exp Bot 55:411–422
Wegner LH, Zimmermann U (1998) Simultaneous recording of xylem pressure and trans-root potential in roots of intact glycophytes by using a novel xylem pressure probe technique. Plant Cell Environ 21:849–865
Westgate M, Steudle E (1985) Water transport in the midrib tissue of maize leaves. Direct measurements of the propagation of changes in cell turgor across a plant tissue. Plant Physiol 78:183–191
Ye Q, Steudle E (2006) Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environ 29:459–470
Ye Q, Wiera B, Steudle E (2004) A cohesion/tension mechanism explains the gating of water channels (aquaporins) in Chara internodes by high concentration. J Exp Bot 55:449–461
Zwieniecki M, Thompson M, Holbrook N (2002) Understanding the hydraulics of porous pipes: tradeoffs between water uptake and root length utilization. J Plant Growth Regul 21:315–323
Zwieniecki MA, Stone H, Leigh A, Boyce CK, Holbrook NM (2006) Hydraulic design of pine needles: one-dimensional optimization for single-veined leaves. Plant Cell Environ 29:803–809
Zwieniecki M, Brodribb T, Holbrook NM (2007) Hydraulic design of leaves: insights from rehydration kinetics. Plant Cell Environ 30:910–921
Acknowledgments
The authors thank Whitney Kress for her valuable assistance in the experiments. We are also grateful to Fulton Rockwell for reading and discussing the manuscript. This work was supported by the National Science Foundation (Grant no.10B-0517071).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Q., Holbrook, N.M. & Zwieniecki, M.A. Cell-to-cell pathway dominates xylem-epidermis hydraulic connection in Tradescantia fluminensis (Vell. Conc.) leaves. Planta 227, 1311–1319 (2008). https://doi.org/10.1007/s00425-008-0703-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0703-7