Abstract.
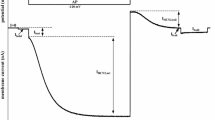
The purpose of the present study was to examine how apamin interacts with the three cloned subtypes of small-conductance Ca2+-activated K+ channels (hSK1, rSK2 and rSK3). Expression of the SK channel subtypes in Xenopus laevis oocytes resulted in large outward currents (0.5–5 µA) after direct injection of Ca2+. In all three cases the Ca2+-activated K+ currents could be totally inhibited by 500 nM apamin. Dose–response curves revealed a subtype-specific affinity for the apamin-induced inhibition with IC50 values of 704 pM and 196 nM (biphasic) for hSK1, 27 pM for rSK2 and 4 nM for rSK3. Consistent with these results, membranes prepared from oocytes expressing the SK channel subtypes bound 125I-labelled apamin with distinct dissociation constants (K d values) of approx. 390 pM for hSK1, 4 pM for rSK2 and 11 pM for rSK3. These results show that apamin binds to and blocks all three subtypes of cloned SK channels, and the distinct values for IC50 and K d suggest that apamin may be useful for determining the expression pattern of SK channel subtypes in native tissue.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received after revision: 29 August 2000
Electronic Publication
Rights and permissions
About this article
Cite this article
Grunnet, M., Jensen, B., Olesen, SP. et al. Apamin interacts with all subtypes of cloned small-conductance Ca2+-activated K+ channels. Pflügers Arch - Eur J Physiol 441, 544–550 (2001). https://doi.org/10.1007/s004240000447
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004240000447