Abstract
Mitochondrial temperature is produced by various metabolic processes inside the mitochondria, particularly oxidative phosphorylation. It was recently reported that mitochondria could normally operate at high temperatures that can reach 50℃. The aim of this review is to identify mitochondrial temperature differences between normal cells and cancer cells. Herein, we discussed the different types of mitochondrial thermosensors and their advantages and disadvantages. We reviewed the studies assessing the mitochondrial temperature in cancer cells and normal cells. We shed the light on the factors involved in maintaining the mitochondrial temperature of normal cells compared to cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mitochondrial structure
Considered the powerhouse of eukaryotic cells, mitochondria are composed of mitochondrial matrix enclosed by an outer mitochondrial membrane (OMM) and intermembrane space enclosed by an inner mitochondrial membrane (IMM) [1, 4].
The structure of mitochondria is not always rigid. They form a highly dynamic network that responds to different stimuli, such as metabolic alterations and apoptosis [81].
Various types of cells require different mitochondrial demands; consequently, variations in mitochondrial structures are associated with multiple functional implications to meet the needs of each cell type. The cristae of the IMM are the sites of the protein complexes responsible for energy and reactive oxygen species (ROS) production [27]. Therefore, cells with greater energy demand have more cristae and less mitochondrial matrix volume. For example, heart cells display more cristae than matrix volume, while the opposite is true for liver cells [10, 58].
Function of the mitochondria in normal cells
Mitochondria have many critical functions in cellular metabolism. Five protein complexes (I to V), constitutively embedded in the IMM and known as super-complexes (SCs), are responsible for the oxidative phosphorylation (OXPHOS) process. Complex I (NADH-Q oxidoreductase) is responsible for NADH oxidation and proton transfer to the intermembrane space. Similarly, complex II (succinate dehydrogenase) oxidizes FADH2. Afterward, complexes I and II transfer their electrons to coenzyme Q (CoQ) (also known as ubiquinone), resulting in its reduction into ubiquinol. Ubiquinol then transfers its electrons to complex III (ubiquinol-cytochrome c oxidoreductase), which contributes to pumping more protons to the intermembrane space. Electrons from complex III are transferred to complex IV (cytochrome c oxidase) by cytochrome c (Cyt c) protein. Concomitantly, more H+ protons are transferred, and molecular oxygen is reduced into water. Finally, complex V (F0F1 ATP synthase) takes advantage of the developed proton motive force (PMF), in which the protons H+ re-enters through complex V to synthesize ATP from ADP [5], as shown in Fig. 1.
Electron cryotomography studies showed that the ETC protein complexes (I, II, III, IV) are found in less curved regions in the cristae, while F0F1 ATP synthases are found in tightly curved regions of the crista. Therefore, cells with high energy demand will have more tightly curved regions due to the importance of ATP synthases. For example, skeletal and cardiac muscles have more curved cristae than brown adipose cells to sustain their contractile ability [59]. On the other hand, brown adipose cells need less curved cristae due to the presence of more ETC complexes, since their energy is dissipated via a mechanism known as “uncoupling” [22, 37].
Mitochondrial functions are not limited to cellular metabolism or ATP production. Mitochondria initiate retrograde signaling to stimulate the nucleus to activate ATP production signaling pathways. This is achieved by activating the mitostress signals, via producing ROS and decreasing the inner mitochondrial membrane potential [33]. Also, mitochondria maintain cellular homeostasis by reducing ROS and H2O2 using antioxidant mechanisms, such as manganese-dependent superoxide dismutase and glutathione peroxidase [30, 55]. Importantly, mitochondria orchestrate the adaptive immunity of the cells by releasing mitochondrial antiviral signaling protein (MAVS), damage-associated molecular pattern protein (DAMP), and mitochondrial ROS [14, 48].
Function of the mitochondria in cancer cells
For decades, cancer cells were thought to be defective in the mitochondrial respiration, and to undergo a high glycolysis rate to meet their energy requirements [28]. The dogma of cancer metabolism however has evolved, and today it appears that both glycolysis and mitochondrial metabolism are critical for tumor growth [78]. This is in accordance with Donnelly and Scheffler [24] who showed that cancer cells increase the rate of glycolysis and citric acid cycle (CAC) to maintain a constant amount of produced energy (∆GATP).
Glycolysis replenishes the metabolites required for nucleotide synthesis and antioxidant activity through pentose phosphate pathway (PPP), NADPH production, nucleotide synthesis, and methylation reactions through one-carbon metabolism [21, 32]. Mitochondrial metabolism is crucial for tumor growth. Krebs cycle intermediates replenish the metabolites required for the synthesis of nucleotides, lipids, amino acids, and heme, all of which are required for the biosynthesis of new cells [82]. Cancer cells can replace the carbon used in the biosynthesis process by oxidizing glutamine to replenish α-ketoglutarate, branched-chain amino acid to replenish succinyl-CoA, pyruvate carboxylase to replenish oxaloacetate, and ubiquinol to replenish ubiquinone [20, 45]. This may reflect the importance of mitochondria for cancer cells to synthesize macromolecules and produce energy [83]. This was also confirmed by the importance of mitochondrial genome integrity and retention of function for cellular malignancy [36, 82]. This opposes the hypothesis of inefficient mitochondria in cancer cells as an explanation of Warburg’s effect that states that cancer cells shift their metabolism from oxidative phosphorylation to glycolytic pathways to meet their high energy demand.
The importance of mitochondrial temperature
Different cellular dysfunctions and physiological and pathological conditions lead to altered energy metabolism and mitochondrial energetics [35], leading to a change in the mitochondrial temperature [69]. This is why mitochondrial temperature is an important indicator and differentiates between normal and dysfunctional cells [35]. One of the most prominent applications of mitochondrial thermometry is mitochondria-targeted drug delivery, using thermoresponsive nanocarriers. These nanocarriers are able to enhance the accumulation of anticancer drugs in mitochondria of specific endogenous mitochondrial temperature. This increases the selectivity to cancer cells and contributes to the reversal of drug resistance in cancer [63].
Sources of mitochondrial temperature
It was long believed that the primary source of mitochondrial temperature is mitochondrial uncoupling (Fig. 2B). ATP production is not 100% efficient since some H+ ions escape ATP synthase that converts ADP to ATP in the mitochondrial coupling, resulting in its return to the mitochondrial matrix via uncoupling proteins (UCPs) (Fig. 2). This process is the mitochondrial uncoupling that causes energy dissipation in the form of heat, and results in a transient increase in cellular temperature [2, 7, 8, 26, 40, 61, 67].
Mitochondrial coupling and uncoupling. A Mitochondrial coupling: proton pumps of the electron transport chain uses redox energy to generate proton motive force. This force will regenerate ATP by ATP synthase. B Mitochondrial uncoupling inhibits the coupling between electron transport and ATP-synthetic reactions. This in turn causes loss of the energy as heat. Created with BioRender.com
The increase in mitochondrial temperature due to uncoupling was shown upon treating human cervical cancer HeLa cells by carbonyl cyanide-4 trifluoromethoxy phenylhydrazone (FCCP) (mitochondrial oxidative phosphorylation uncoupler). The mitochondrial temperature increased from 32.6 to 35 °C with a concomitant decrease in the fluorescence intensity of the ATP sensing probe. This effect was abolished after removing FCCP [60]. It was also shown that there is a heterogeneity in the intra-cellular temperature, and that the mitochondrial temperature is no exception, as it follows the thermal diffusion equation [17, 38, 50, 64, 80].
Methods to assess mitochondrial temperature
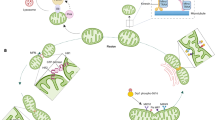
Novel tools were considerably developed over the past decade to assess mitochondrial heat production, most notably by fluorescent thermometry. One of the ways to detect mitochondrial temperature change is to detect the effect of uncoupling on mitochondrial temperature. Uncouplers inhibit the coupling between electron transport and ATP-synthetic (phosphorylation) reactions. This in turn inhibits ATP synthesis, and instead, causes loss of the energy as heat [16, 70] (Fig. 3). A fluorescent nanogel was first used to detect an increase of ≈1 °C in African green monkey kidney fibroblast-like cells (COS-7) upon uncoupling [29]. The nanogel was later enhanced into a fluorescent polymeric thermometer (FTP), with higher accuracy and resolution. This was achieved using time-correlated single-photon counting system–based fluorescence lifetime imaging (FLIM) to image the localization of FTP in COS-7 cells, and estimate temperature change accordingly [53]. Although the average cellular temperature increased by around 1 °C, it was difficult to attribute the increase in temperature to mitochondrial heat production as the probes were localized in the cytoplasm, not specifically mitochondria [44].
Measuring mitochondrial temperature by fluorescent thermosensors. a Cells are stained by fluorescent thermosensors and gradually heated. b Fluorescence intensity, measured by means of fluorescent microscopy at different temperatures will be recorded. c A plot of fluorescence intensity versus temperature will be drawn “Calibration plot.” d Uncoupler reagent (such as FCCP) will be added to induce mitochondrial uncoupling. e By means of fluorescence microscope, the fluorescence intensity of the cells will be measured by fluorescence microscopy and plotted (c) to conclude the cell temperature
Other fluorescent thermosensors localized to the mitochondria were developed by including rhodamine-CS-NIR-based Mito-RTP dye [34] and rosamine-compound-based MTY dye [3, 17]. Upon FCCP treatment of HeLa cells, Mito-RTP dye reflected an increase in mitochondrial temperature by an estimated 3.5–4 °C [34, 44]. Upon FCCP treatment, temperature sensing (7-(diethylamino) coumarin-3-carbaldehyde dye) and ATP sensing probe (rhodamine B derivative) reflected an increase in HeLa cells by 2.4 °C [60]. MTY showed an increase in mitochondrial temperature with increased respiratory activity in HEK 293 cells and primary skin fibroblasts, by up to 10 °C above that of the culture medium (38 °C) [17].
However, fluorescent thermosensors do not directly measure the temperature; they depend on the binding of the mitochondrial membrane (negatively charged) to the probe (positively charged) [60]. Thus, the change of the mitochondrial potential can change the localization of the dyes and can cause fluorescence quenching [18]. Also, fluorescent thermosensors are prone to biases in different cell types, metabolic status [39], and environmental conditions such as pH, viscosity, and ionic strength [44]. For example, Chrétien et al. [18] showed that MTY can measure the mitochondrial temperature of HEK and HeLa cells, but not the primary fibroblasts cells. This is why all data related to the environmental conditions must be reported, and purified probes must be used to study the effect of these parameters on the temperature measurement [44]. Moreover, the mitochondrial compartment in which the temperature was measured must not be extrapolated to the whole mitochondria without taking into consideration all the previous factors [18]. Thus, precise calibration in the extracellular environment and accurate colocalization of organelles with mitochondrial targeting dye are required for accurate mitochondrial temperature measurement [23]. Because of all the aforementioned limitations of these sensors, there is an urge to develop a stable and unbiased mitochondrial temperature assessment technique [18].
Other types of thermosensors that can localize inside the mitochondrial matrix are genetically encoded fluorescent proteins such as tsGFP [41], gTEMP [50], and emGFP [64]. When HeLA cells were treated with the uncoupler FCCP, fluorescence was detected by confocal, epifluorescence, and confocal fluorescence microscopy, respectively. tsGFP1 and gTEMP showed an estimated 6 °C increase in mitochondrial temperature [50], while emGFP reflected an estimated increase of 3–5 °C [44, 64]. Moreover, by means of these thermos-sensors, HELA cells’ mitochondria were shown to have different PMFs, and hence different intra-cellular temperatures. This feature is known as “thermal heterogeneity” and it has two levels: intra-cellular and inter-cellular. This is crucial for cell–cell behavior differences [6].
Mitochondrial temperature in normal cells
Chrétien et al. [17] showed that the temperature of active mitochondria is 10℃ warmer than its normal physiological temperature due to the activity of respiratory chain (RC) complexes . Those were shown to reach their maximum potential at 48℃ in human embryonic kidney cells HEK 293, and in primary skin fibroblasts [17]. This observation was further confirmed by the inhibition of the RC complexes that lead to the decrease of the mitochondrial temperature from 50 to 38 °C [17]. In another set of experiments, Okabe et al. [53] measured the mitochondrial temperature of COS-7 cells using FTP and FLIM. They showed that the mitochondrial temperature was ranging from 43 to 48 °C.
The high mitochondrial temperature was attributed to the maximal respiratory chain complex activities [17]. This was supported by the enrichment of heat shock proteins and thermoprotectant solutes that protect the protein structures in high temperatures [18], and by the overexpression of the mitochondrial uncoupling protein UCP2 in liver cancer cells [11, 13, 15, 25]. This was confirmed by knocking down UCP2 expression in human hepatic cancer HEPG2 cells, leading to an increase in the mitochondrial membrane potential and ATP/ADP ratio, and a corresponding decrease in mitochondrial temperature [75]. Further experiments showed that peroxisome proliferator-activated receptor α (PPAR), the activator of UCP2, was overexpressed in liver cancer cells compared to normal liver cells [13, 46, 51, 56, 71].
Mitochondrial temperature in cancer cells
As discussed previously, Warburg’s effect suggests that the cancer cells are defective in the mitochondrial respiration and must undergo high glycolysis in order to meet their energy supply requirements [28]. This implicates that the mitochondrial temperature in cancer cells is expected to be lower than that of normal cells. On the other hand, uncoupling is an important feature for cancer initiation, progression, metabolic adaptation, and drug chemoresistance [9, 73]. This implicates that the mitochondrial temperature in cancer cells is higher than that of normal cells.
Several reports confirmed that the mitochondrial temperature in cancer cells is higher than that in normal cells. Ruan et al. [63] showed that the mitochondrial temperature in murine breast cancer cells is about 48 °C. This was experimentally confirmed by showing that the highest release of the anticancer drug PTX loaded on thermoresponsive nanocarrier (poly N-isopropyl acrylamide, PNIPAM) was achieved when the lower critical solution temperature was adjusted to ~ 48◦C [62, 63]. This was assessed in murine breast cancer 4T1 cells at 48 °C compared to 37 °C and 25 °C [62]. Later, Wang et al. [76] also showed that the mitochondrial temperature was higher in murine cancer cells than in normal cells. The researchers used MTY dye staining of murine bladder cancer MB49 cells and murine RAW 264.7 macrophages. MB49 cells showed lower fluorescence of the mitochondria-targeted probe when compared with the RAW 264.7 cells, and correspondingly, a higher temperature. Moreover, Wang et al. [76] showed that the mitochondrial temperature in murine MB49 cancer cells was higher than that of human umbilical vein endothelial cells (HUVEC). However, we cannot find this conclusive due to species difference between the two cell lines.
Arai et al. [3] showed that the mitochondrial temperature of human lung carcinoma cell line H69AR was higher than that of HUVEC cells also using MTY dye [63]. However, Arai’s team was comparing malignant epithelial cells to normal endothelial cells and there was no proof that mitochondrial temperature difference is similar in both cell types. Importantly, the MTY assessment of mitochondrial temperature must be taken cautiously since, as previously mentioned, it is affected by the change in environmental factors and the affinity of the targets to the dye [18, 44]. The higher mitochondrial temperature in cancer cells on the other hand may be supported by the studies that showed that the mitochondrial respiration rate increases in cancer cells [74, 82].
Qiao et al. [60] measured the mitochondrial temperature in HeLA cells using PNIPAm-VBC-DACC-CTPP as a temperature sensor and RhB-ABA as an ATP sensor. From Fig. 4, we can conclude that HeLA’s mitochondrial temperature was 35–36 °C. Di et al. [23] assessed the mitochondrial temperature in HeLa cells using lanthanide-doped upconversion nanoparticles covalently linked to the mitochondria-targeting moiety (3 carboxypropyl) triphenylphosphonium bromide (TPP). From Fig. 5, we can conclude that the mitochondrial temperature of HeLa cells was 32–33 °C [23]. However, since these studies did not compare cancer to normal epithelial cells, there was no conclusive evidence that the mitochondrial temperature is higher or lower in cancer compared to normal cells. Indeed, comparing the mitochondrial temperature in those studies to that of normal epithelial cells in Chrétien et al. study suggests that the mitochondrial temperature in cancer cells is lower than that of normal cells. Still, caution must be taken since there is no standardized method to precisely measure the mitochondrial temperature, especially considering the limitations of MTY dye.
Mitochondrial temperature fluctuation response to FCCP inhibition in HeLa cells. Live HeLa cells were prestained with the T sensing probe (0.5 mg mL−1, 20 min) and the ATP sensing probe (5.0 μM, 20 min). The intensity data was obtained from 25 live HeLa cells. Reprinted with permission from “Qiao, J., Chen, C., Shangguan, D., Mu, X., Wang, S., Jiang, L., & Qi, L. (2018). Simultaneous monitoring of mitochondrial temperature and ATP fluctuation using fluorescent probes in living cells. Analytical chemistry, 90(21), 12,553–12,558”, Fig. 6A. Copyright 2022 American Chemical Society
Visualization of mitochondrial thermal dynamics in HeLa cells response to glucose stimulations. (Left) Upconversion nanoparticles (UCNPs) at (3carboxypropyl) triphenylphosphonium bromide (TPP) images. MitoTracker (red) and UCNPs@TPP (green) from different treatments. (Middle) Mitochondrial temperature dynamics in the presence of 5 mg/mL glucose within 30 min. (Right) Student’s t test of both no glucose and glucose at 10 min (p < 0.0001). Reprinted with permission from “Di, X., Wang, D., Zhou, J., Zhang, L., Stenzel, M. H., Su, Q. P., & Jin, D. (2021). Quantitatively monitoring in situ mitochondrial thermal dynamics by upconversion nanoparticles. Nano letters, 21(4), 1651–1658”, Fig. 4A. Copyright 2022 American Chemical Society
Factors regulating mitochondrial temperature
Mitochondrial membranes
Mitochondrial membranes play an important role in the regulation of mitochondrial temperature. The composition of the outer and inner mitochondrial membranes and their different protein-to-lipid ratios are primarily responsible for maintaining the mitochondrial temperature and providing layers of thermal insulation. The mitochondrial matrix is considered a narrow space flanked by heat-producing membranes to maintain the temperature difference between the cytosol and the mitochondria [17]. The cytosol contains mitochondrial domains to minimize heat conduction to the rest of the cell, as reported in HEK293 cells [17]. Recent research showed how the various composition of lipids in the different mitochondrial membranes affects the thermal stability of the RC complexes and the mitochondrial properties in response to temperature variation, such as swelling and permeability transition [17]. A study on liver mitochondria reported that the increase in membrane conductance due to hyperthermia was related to a sudden transformation in the order of the inner mitochondrial membrane [79].
Heat shock proteins (HSPs)
Mitochondrial HSPs (mt-HSPs) were proposed to protect the mitochondria and keep their integrity under high mitochondria temperature [17, 52]. Seventy kDa HSP family (mt-HSP70) is remarkably abundant in the mitochondria of prokaryotes and eukaryotes [43]. HSPs are thought to play a role in protecting mt-DNA at relatively high temperatures [52]. This is extremely important since mt-DNA is not protected by a coat of histone proteins, rendering it more vulnerable to environmental insults such as heat and ROS damage [31]. This was also confirmed by the loss of mt-DNA function using mutant S. cerevisiae that do not express mt-HSP70 or mt-HSP78 [47]. Similarly, mt-HSP70 and mt-HSP40 contribute to maintaining mt-DNA structure and replication in human-pathogenic protist Trypanosoma brucei and yeast strains [19, 72].
Indeed, HSPs contribute to thermotolerance and thermoregulation by decreasing the thermally induced ROS oxidative damage especially that mitochondria are the main source of ROS [12, 31, 49, 52, 54, 68]
FMC1 gene
The formation of the mitochondrial complex V assembly factor 1 homolog (FMC1) gene plays a critical role in maintaining the thermal stability of ATP synthase in yeast. This is accomplished by retaining the assembly and stability of the F1 part of ATP synthase, responsible for ATP synthesis, at high temperatures [42, 57, 65, 77]. C7orf55 is the homolog of the FMC1 gene in humans. Further research is needed to assess the extent of its contribution in maintaining the thermal stability of ATP synthase in human cells.
Conclusion
Mitochondrial temperature is an essential indicator of mitochondrial function. Credible data suggest that cancer mitochondrial temperature is higher than that of normal cells. This finding can be of significance in the development of cancer biomarkers, specific anticancer drug delivery, and drug release from their conjugates. However, there is an urge to standardize the methods of measuring the mitochondrial temperature to be able to compare the results of different studies. Still, more comparative studies between normal and cancer cells of the same types are required to be able to identify whether the mitochondrial temperature can be used as a cancer biomarker.
Data availability
All data in this study are included in this manuscript.
References
Alberts B (2018). Molecular biology of the cell.
Andrews ZB, Diano S, Horvath TL (2005) Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat Rev Neurosci 6(11):829–840
Arai S, Suzuki M, Park S-J, Yoo JS, Wang L, Kang N-Y, Ha H-H, Chang Y-T (2015) Mitochondria-targeted fluorescent thermometer monitors intracellular temperature gradient. Chem Commun 51(38):8044–8047. https://doi.org/10.1039/c5cc01088h
Archibald JM (2011) Origin of eukaryotic cells: 40 years on. Symbiosis 54(2):69–86
Arulkumaran N, Deutschman CS, Pinsky MR, Zuckerbraun B, Schumacker PT, Gomez H, Gomez A, Murray P, Kellum JA, Workgroup AX (2016) Mitochondrial function in sepsis SHOCK 45(3):271–281. https://doi.org/10.1097/shk.0000000000000463
Aryaman J, Johnston IG, Jones NS (2019) Mitochondrial heterogeneity. Front Genet 9:718. https://doi.org/10.3389/fgene.2018.00718
Baffou G (2014) A critique of methods for temperature imaging in single cells. Nat Methods 11(9):899–901
Baffou G (2015) Reply to: “Validating subcellular thermal changes revealed by fluorescent thermosensors” and “The 10 5 gap issue between calculation and measurement in single-cell thermometry.” Nat Methods 12(9):803–803
Baffy G (2017) Mitochondrial uncoupling in cancer cells: liabilities and opportunities. Biochimica et Biophysica Acta (BBA) Bioenergetics 1858(8):655–664. https://doi.org/10.1016/j.bbabio.2017.01.005
Bochimoto H, Matsuno N, Ishihara Y, Shonaka T, Koga D, Hira Y, Nishikawa Y, Furukawa H, Watanabe T (2017) The ultrastructural characteristics of porcine hepatocytes donated after cardiac death and preserved with warm machine perfusion preservation. PLoS One 12(10):e0186352
Busquets S (1998) In the rat, tumor necrosis factor α administration results in an increase in both UCP2 and UCP3 mRNAs in skeletal muscle: a possible mechanism for cytokine-induced thermogenesis? FEBS Lett 440(3):348–350
Cabiscol E (2002) Mitochondrial Hsp60, resistance to oxidative stress, and the labile iron pool are closely connected in Saccharomyces cerevisiae. J Biol Chem 277(46):44531–44538
Carretero MV (1998) Transformed but not normal hepatocytes express UCP2. FEBS Lett 439(1–2):55–58
Chandel NS (2014) Mitochondria as signaling organelles. BMC Biol 12:34
Chavin KD (1999) Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem 274(9):5692–5700
Chouchani ET, Kazak L, Spiegelman BM (2018) New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metab 29(1):27–37. https://doi.org/10.1016/j.cmet.2018.11.002
Chrétien D, Bénit P, Ha H-H, Keipert S, El-Khoury R, Chang Y-T, Jastroch M, Jacobs HT, Rustin P, Rak M (2018) Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol 16(1):e2003992. https://doi.org/10.1371/journal.pbio.2003992
Chrétien D, Bénit P, Leroy C, El-Khoury R, Park S, Lee JY, Chang Y-T, Lenaers G, Rustin P, Rak M (2020) Pitfalls in monitoring mitochondrial temperature using charged thermosensitive fluorophores. Chemosensors 8(4):124. https://doi.org/10.3390/chemosensors8040124
Ciesielski GL (2013). Nucleoid localization of Hsp40 Mdj1 is important for its function in maintenance of mitochondrial DNA. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1833(10), 2233–2243.
Cluntun AA, Lukey MJ, Cerione RA, Locasale JW (2017) Glutamine metabolism in cancer: understanding the heterogeneity. Trends in Cancer 3(3):169–180. https://doi.org/10.1016/j.trecan.2017.01.005
DeBerardinis RJ, Chandel NS (2016) Fundamentals of cancer metabolism. Science. Advances 2(5):e1600200. https://doi.org/10.1126/sciadv.1600200
de-Lima-Júnior, J. C. (2019) Abnormal brown adipose tissue mitochondrial structure and function in IL10 deficiency. EBioMedicine 39:436–447
Di X, Wang D, Zhou J, Zhang L, Stenzel MH, Su QP, Jin D (2021) Quantitatively monitoring in situ mitochondrial thermal dynamics by upconversion nanoparticles. Nano Lett 21(4):1651–1658. https://doi.org/10.1021/acs.nanolett.0c04281
Donnelly M, Scheffler IE (1976) Energy metabolism in respiration-deficient and wild type Chinese hamster fibroblasts in culture. J Cell Physiol 89(1):39–51
Faggioni R (1998) Induction of UCP2 gene expression by LPS: a potential mechanism for increased thermogenesis during infection. Biochem Biophys Res Commun 244(1):75–78
Fahimi P (2020) A note on the consequences of a hot mitochondrion: some recent developments and open questions. Бioфiзичний вicник 2020(43):14–21
Glancy B (2020) The functional impact of mitochondrial structure across subcellular scales. Frontiers in Physiology 11:1462
Gogvadze V, Orrenius S, Zhivotovsky B (2008) Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 18(4):165–173
Gota C, Okabe K, Funatsu T, Harada Y, Uchiyama S (2009) Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J Am Chem Soc 131(8):2766–2767. https://doi.org/10.1021/ja807714j
Guo CH, Che PC (2012) Mitochondrial free radicals, antioxidants, nutrient substances, and chronic hepatitis C. In: Antioxidant Enzyme, pp 237–264
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press
Heiden MGV, DeBerardinis RJ (2017) Understanding the intersections between metabolism and cancer biology. Cell 168(4):657–669. https://doi.org/10.1016/j.cell.2016.12.039
Herst PM (2017). Functional mitochondria in health and disease. Front Endocrinol (Lausanne.
Homma M, Takei Y, Murata A, Inoue T, Takeoka S (2015) A ratiometric fluorescent molecular probe for visualization of mitochondrial temperature in living cells. Chem Commun 51(28):6194–6197. https://doi.org/10.1039/c4cc10349a
Jonckheere AI, Smeitink JAM, Rodenburg RJT (2012) Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis 35(2):211–225. https://doi.org/10.1007/s10545-011-9382-9
Ju YS (2014) Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. Elife 3:02935
Kang HW (2009) Mice lacking Pctp /StarD2 exhibit increased adaptive thermogenesis and enlarged mitochondria in brown adipose tissue. J Lipid Res 50(11):2212–2221
Kang J-S (2018) Theoretical model and characteristics of mitochondrial thermogenesis. Biophysics Reports 4(2):63–67. https://doi.org/10.1007/s41048-018-0054-2
Kim YK, Lee J, Bi X, Ha H, Ng SH, Ahn Y, Lee J, Wagner BK, Clemons PA, Chang Y (2011) The binding of fluorophores to proteins depends on the cellular environment. Angew Chem 123(12):2813–2815. https://doi.org/10.1002/ange.201007626
Kiyonaka S (2015) Validating subcellular thermal changes revealed by fluorescent thermosensors. Nat Methods 12(9):801–802
Kiyonaka S, Kajimoto T, Sakaguchi R, Shinmi D, Omatsu-Kanbe M, Matsuura H, Imamura H, Yoshizaki T, Hamachi I, Morii T, Mori Y (2013) Genetically encoded fluorescent thermosensors visualize subcellular thermoregulation in living cells. Nat Methods 10(12):1232–1238. https://doi.org/10.1038/nmeth.2690
Lefebvre-Legendre L (2001) Identification of a nuclear gene (FMC1) required for the assembly/stability of yeast mitochondrial F1-ATPase in heat stress conditions. J Biol Chem 276(9):6789–6796
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22(1):631–677
Macherel D, Haraux F, Guillou H, Bourgeois O (2021) The conundrum of hot mitochondria. Biochim Biophys Acta 1862(2):148348. https://doi.org/10.1016/j.bbabio.2020.148348
Martínez-Reyes I, Cardona LR, Kong H, Vasan K, McElroy GS, Werner M, Kihshen H, Reczek CR, Weinberg SE, Gao P, Steinert EM, Piseaux R, Budinger GRS, Chandel NS (2020) Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature 585(7824):288–292. https://doi.org/10.1038/s41586-020-2475-6
Memon RA (2000) Up-regulation of peroxisome proliferator-activated receptors (PPAR-α) and PPAR-γ messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPAR-γ-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology 141(11):4021–4031
Moczko M (1995) The mitochondrial ClpB homolog Hsp78 cooperates with matrix Hsp70 in maintenance of mitochondrial function. J Mol Biol 254(4):538–543
Mohanty A, Tiwari-Pandey R, Pandey NR (2019) Mitochondria: the indispensable players in innate immunity and guardians of the inflammatory response. J Cell Commun Signal 13(3):303–318
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13
Nakano M, Arai Y, Kotera I, Okabe K, Kamei Y, Nagai T (2017) Genetically encoded ratiometric fluorescent thermometer with wide range and rapid response. PLoS ONE 12(2):e0172344. https://doi.org/10.1371/journal.pone.0172344
Nakatani T (2002) Mechanism for peroxisome proliferator-activated receptor-α activator-induced up-regulation of UCP2 mRNA in rodent hepatocytes. J Biol Chem 277(11):9562–9569
Nasr MA, Dovbeshko GI, Bearne SL, El-Badri N, Matta CF (2019) Heat shock proteins in the “hot” mitochondrion: identity and putative roles. BioEssays 41(9):1900055. https://doi.org/10.1002/bies.201900055
Okabe K, Inada N, Gota C, Harada Y, Funatsu T, Uchiyama S (2012) Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat Commun 3(1):705. https://doi.org/10.1038/ncomms1714
Oksala NK (2014) Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Biol 3:25–28
Osellame LD, Blacker TS, Duchen MR (2012) Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab 26(6):711–723
Patsouris D (2006) Peroxisome proliferator-activated receptor α mediates the effects of high-fat diet on hepatic gene expression. Endocrinology 147(3):1508–1516
Pedersen PL (1996) Frontiers in ATP synthase research: understanding the relationship between subunit movements and ATP synthesis. J Bioenerg Biomembr 28(5):389–395
Phillips D (2012) Regulation of oxidative phosphorylation complex activity: effects of tissue-specific metabolic stress within an allometric series and acute changes in workload. American J Physiology-Regulatory, Integr and Comp Physiol 302(9):1034–1048
Picard M (2015) Trans-mitochondrial coordination of cristae at regulated membrane junctions. Nat Commun 6(1):6259
Qiao J, Chen C, Shangguan D, Mu X, Wang S, Jiang L, Qi L (2018) Simultaneous monitoring of mitochondrial temperature and ATP fluctuation using fluorescent probes in living cells. Anal Chem 90(21):12553–12558. https://doi.org/10.1021/acs.analchem.8b02496
Rajagopal MC (2019) Transient heat release during induced mitochondrial proton uncoupling. Communications Biology 2(1):1–6. https://doi.org/10.1038/s42003-019-0535-y
Ruan L (2019) Thermoresponsive drug delivery to mitochondria in vivo. Chem Commun 55(97):14645–14648. https://doi.org/10.1039/c9cc07538k
Ruan L, Chen J, Du C, Lu H, Zhang J, Cai X, Dou R, Lin W, Chai Z, Nie G, Hu Y (2021) Mitochondrial temperature-responsive drug delivery reverses drug resistance in lung cancer. Bioact Mater 13:191–199
Savchuk OA, Silvestre OF, Adão RMR, Nieder JB (2019) GFP fluorescence peak fraction analysis based nanothermometer for the assessment of exothermal mitochondria activity in live cells. Sci Rep 9(1):7535. https://doi.org/10.1038/s41598-019-44023-7
Schwimmer C (2005) Increasing mitochondrial substrate-level phosphorylation can rescue respiratory growth of an ATP synthase-deficient yeast. J Biol Chem 280(35):30751–30759
Stewart JB (2015) Simultaneous DNA and RNA mapping of somatic mitochondrial mutations across diverse human cancers. PLoS Genet 11(6):1005333
Suzuki M (2015) The 10 5 gap issue between calculation and measurement in single-cell thermometry. Nat Methods 12(9):802–803
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126(1):45–51
Tang W, Gao H, Li J, Wang X, Zhou Z, Gai L, Feng XJ, Tian J, Lu H, Guo Z (2020). A general strategy for the construction of NIR‐emitting Si‐rhodamines and their application for mitochondrial temperature visualization. Chemistry – An Asian Journal, 15(17), 2724–2730. https://doi.org/10.1002/asia.202000660
Terada H (1990) Uncouplers of oxidative phosphorylation. Environ Health Perspect 87:213–218. https://doi.org/10.1289/ehp.9087213
Tsuboyama-Kasaoka N (1999) Up-regulation of liver uncoupling protein-2 mRNA by either fish oil feeding or fibrate administration in mice. Biochem Biophys Res Commun 257(3):879–885
Týč J, Klingbeil MM, & Lukeš J (2015). Mitochondrial heat shock protein machinery hsp70/hsp40 is indispensable for proper mitochondrial DNA maintenance and replication. MBio, 6(1).
Valle A, Oliver J, Roca P (2010) Role of uncoupling proteins in cancer. Cancers 2(2):567–591. https://doi.org/10.3390/cancers2020567
Viale A, Corti D, Draetta GF (2015) Tumors and mitochondrial respiration: a neglected connection. Can Res 75(18):3687–3691. https://doi.org/10.1158/0008-5472.can-15-0491
Vozza A (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci 111(3):960–965
Wang D, Zhou M, Huang H, Ruan L, Lu H, Zhang J, Chen J, Gao J, Chai Z, Hu Y (2019) Gold nanoparticle-based probe for analyzing mitochondrial temperature in living cells. ACS Appl Bio Mater 2(8):3178–3182. https://doi.org/10.1021/acsabm.9b00463
Weber J, & Senior AE (1997). Catalytic mechanism of F1-ATPase. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1319(1), 19–58.
Weinberg SE, Chandel NS (2015) Targeting mitochondria metabolism for cancer therapy. Nat Chem Biol 11(1):9–15. https://doi.org/10.1038/nchembio.1712
Willis W (2000) Hyperthermia impairs liver mitochondrial function in vitro. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 278(5):1240–1246
Xie T-R, Liu C-F, Kang J-S (2017) Dye-based mito-thermometry and its application in thermogenesis of brown adipocytes. Biophysics Reports 3(4):85–91. https://doi.org/10.1007/s41048-017-0039-6
Yu R (2020). Regulation of mammalian mitochondrial dynamics: opportunities and challenges. Frontiers in Endocrinology, 11(374).
Zong W-X, Rabinowitz JD, White E (2016) Mitochondria and cancer. Mol Cell 61(5):667–676. https://doi.org/10.1016/j.molcel.2016.02.011
Zu XL, Guppy M (2004) Cancer metabolism: facts, fantasy, and fiction. Biochem Biophys Res Commun 313(3):459–465
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was funded by the Academy of Scientific Research and Technology, Egypt, ASRT grant #5275 (JESOR).
Author information
Authors and Affiliations
Contributions
All authors listed (Zaynab El-Gammal, Mohamed A. Nasr, Ahmed O. Elmehrath, Radwa A. Salah, Shams M. Saad, Nagwa El-Badri) have contributed sufficiently to the project to be included as authors, and all those who are qualified to be authors are listed in the author byline.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Human and animal ethics
Not applicable.
Consent for publication
The authors warrant that:
-
This manuscript has not been published before in any form.
-
This manuscript is not being concurrently submitted to and is not under consideration by another publisher.
-
The authors listed above are listed in the proper order and that no author entitled to credit has been omitted.
-
This manuscript does not libel anyone, infringe anyone’s copyright, or otherwise violate anyone’s statutory or common law rights.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Gammal, Z., Nasr, M.A., Elmehrath, A.O. et al. Regulation of mitochondrial temperature in health and disease. Pflugers Arch - Eur J Physiol 474, 1043–1051 (2022). https://doi.org/10.1007/s00424-022-02719-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-022-02719-2