Abstract
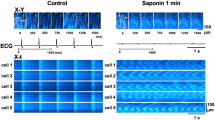
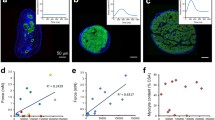
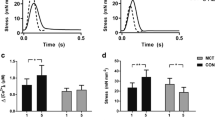
In diseased hearts, impaired muscle within the hearts is passively stretched by contractions of the more viable neighboring muscle during the contraction phase. We investigated whether in the myocardium with nonuniform contraction such passive stretch regionally generates ROS within the stretched region and exacerbates arrhythmias. In trabeculae from rat hearts, force, intracellular Ca2+, and membrane potential were measured. To assess regional ROS generation, the slope of the change in the 2′,7′-dichlorofluorescein fluorescence (DCFslope) was calculated at the each pixel position along the long axis of trabeculae using DCF fluorescence images. Ca2+ waves and arrhythmias were induced by electrical stimulation. A H2O2 (1 mmol/L) jet regionally increased the DCFslope within the jet-exposed region. A blebbistatin (10 μmol/L) jet caused passive stretch of the muscle within the jet-exposed region during the contraction phase and increased the DCFslope within the stretched region, the velocity of Ca2+ waves, and the number of beats after electrical stimulation (0.2 μmol/L isoproterenol), while 3 μmol/L diphenyleneiodonium (DPI), NADPH oxidase inhibitor, decreased them. A jet of a solution containing 0.2 mmol/L H2O2 in addition to 10 µmol/L blebbistatin also increased them. A H2O2 jet within the region where Ca2+ waves propagated increased their velocity. In the myocardium with nonuniform contraction, passive stretch of the muscle by contractions of the neighboring muscle regionally increases ROS within the stretched region, and the regional ROS exacerbates arrhythmias by activating the propagation of Ca2+ waves.





Similar content being viewed by others
References
Aldieri E, Riganti C, Polimeni M, Gazzano E, Lussiana C, Campia I, Ghigo D (2008) Classical inhibitors of NOX NAD(P)H oxidases are not specific. Curr Drug Metab 9:686–696
Bäumer AT, Flesch M, Wang X, Shen Q, Feuerstein GZ, Böhm M (2000) Antioxidative enzymes in human hearts with idiopathic dilated cardiomyopathy. J Mol Cell Cardiol 32:121–130. https://doi.org/10.1006/jmcc.1999.1061
Bérubé J, Caouette D, Daleau P (2001) Hydrogen peroxide modifies the kinetics of HERG channel expressed in a mammalian cell line. J Pharmacol Exp Ther 297:96–102
Beuckelmann DJ, Näbauer M, Erdmann E (1992) Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation 85:1046–1055
Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, Niemeyer BA (2011) Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium 50:407–423. https://doi.org/10.1016/j.ceca.2011.07.006
Bovo E, Lipsius SL, Zima AV (2012) Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol 590:3291–3304. https://doi.org/10.1113/jphysiol.2012.230748
Burgoyne JR, Mongue-Din H, Eaton P, Shah AM (2012) Redox signaling in cardiac physiology and pathology. Circ Res 111:1091–1106. https://doi.org/10.1161/CIRCRESAHA.111.255216
Dries E, Bito V, Lenaerts I, Antoons G, Sipido KR, Macquaide N (2013) Selective modulation of coupled ryanodine receptors during microdomain activation of calcium/calmodulin-dependent kinase II in the dyadic cleft. Circ Res 113:1242–1252. https://doi.org/10.1161/CIRCRESAHA.113.301896
Erickson JR, Joiner MLA, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJL, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME (2008) A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133:462–474. https://doi.org/10.1016/j.cell.2008.02.048
Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, de Tombe PP (2008) Blebbistatin: use as inhibitor of muscle contraction. Pflugers Arch 455:995–1005
Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR (2007) Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4:619–626. https://doi.org/10.1016/j.hrthm.2006.12.047
Goldhaber JI, Liu E (1994) Excitation-contraction coupling in single Guinea-pig ventricular myocytes exposed to hydrogen peroxide. J Physiol 477:135–147
Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, Shah AM (2003) Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41:2164–2171
Hinata M, Matsuoka I, Iwamoto T, Watanabe Y, Kimura J (2007) Mechanism of Na+/Ca2+ exchanger activation by hydrogen peroxide in Guinea-pig ventricular myocytes. J Pharmacol Sci 103:283–292
Hudasek K, Brown ST, Fearon IM (2004) H2O2 regulates recombinant Ca2+ channel α1c subunits but does not mediate their sensitivity to acute hypoxia. Biochem Biophys Res Commun 318:135–141
Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A (2000) Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res 86:152–157
Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A (2001) Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88:529–535
Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RAB, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P (2009) Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res 104:787–795. https://doi.org/10.1161/CIRCRESAHA.108.193334
Jeong EM, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, Dudley SC Jr (2012) Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol 52:454–463. https://doi.org/10.1016/j.yjmcc.2011.09.018
Kentish JC, ter Keurs HEDJ, Ricciardi L, Bucx JJ, Noble MI (1986) Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res 58:755–768
Liu T, O’Rourke B (2013) Regulation of the Na+/Ca2+ exchanger by pyridine nucleotide redox potential in ventricular myocytes. J Biol Chem 288:31984–31992. https://doi.org/10.1074/jbc.M113.496588
Maack C, Kartes T, Kilter H, Schäfers HJ, Nickenig G, Böhm M, Laufs U (2003) Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 108:1567–1574. https://doi.org/10.1161/01.CIR.0000091084.46500.BB
Miura M, Boyden PA, ter Keurs HEDJ (1999) Ca2+ waves during triggered propagated contractions in intact trabeculae. Determinants of the velocity of propagation. Circ Res 84:1459–1468
Miura M, Wakayama Y, Endoh H, Nakano M, Sugai Y, Hirose M, ter Keurs HEDJ, Shimokawa H (2008) Spatial non-uniformity of excitation-contraction coupling can enhance arrhythmogenic delayed afterdepolarizations in rat cardiac muscle. Cardiovasc Res 80:55–61. https://doi.org/10.1093/cvr/cvn162
Miura M, Nishio T, Hattori T, Murai N, Stuyvers BD, Shindoh C, Boyden PA (2010) Effect of nonuniform muscle contraction on sustainability and frequency of triggered arrhythmias in rat cardiac muscle. Circulation 121:2711–2717. https://doi.org/10.1161/CIRCULATIONAHA.109.907717
Miura M, Hattori T, Murai N, Nagano T, Nishio T, Boyden PA, Shindoh C (2012) Regional increase in extracellular potassium can be arrhythmogenic due to nonuniform muscle contraction in rat ventricular muscle. Am J Physiol Heart Circ Physiol 302:H2301–H2309. https://doi.org/10.1152/ajpheart.01161.2011
Miura M, Murai N, Hattori T, Nagano T, Stuyvers BD, Shindoh C (2013) Role of reactive oxygen species and Ca2+ dissociation from the myofilaments in determination of Ca2+ wave propagation in rat cardiac muscle. J Mol Cell Cardiol 56:97–105. https://doi.org/10.1016/j.yjmcc.2012.12.011
Miura M, Taguchi Y, Nagano T, Sasaki M, Handoh T, Shindoh C (2015) Effect of myofilament Ca2+ sensitivity on Ca2+ wave propagation in rat ventricular muscle. J Mol Cell Cardiol 84:162–169. https://doi.org/10.1016/j.yjmcc.2015.04.027
Myerburg RJ, Interian A, Mitrani RM, Kessler KM, Castellanos A (1997) Frequency of sudden cardiac death and profiles of risk. Am J Cardiol 80:10F–19F
Packer M (1985) Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation 72:681–685
Petroff MGV, Kim SH, Pepe S, Dessy C, Marbán E, Balligand JL, Sollott SJ (2001) Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat Cell Biol 3:867–873. https://doi.org/10.1038/ncb1001-867
Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB (2001) Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res 89:453–460
Prosser BL, Ward CW, Lederer WJ (2011) X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333:1440–1445. https://doi.org/10.1126/science.1202768
Siogas K, Pappas S, Graekas G, Goudevenos J, Liapi G, Sideris DA (1998) Segmental wall motion abnormalities alter vulnerability to ventricular ectopic beats associated with acute increases in aortic pressure in patients with underlying coronary artery disease. Heart 79:268–273
Song Y, Shryock JC, Wagner S, Maier LS, Belardinelli L (2006) Blocking late sodium current reduces hydrogen peroxide-induced arrhythmogenic activity and contractile dysfunction. J Pharmacol Exp Ther 318:214–222. https://doi.org/10.1124/jpet.106.101832
Sugai Y, Miura M, Hirose M, Wakayama Y, Endoh H, Nishio T, Watanabe J, ter Keurs HE, Shirato K, Shimokawa H (2009) Contribution of Na+/Ca2+ exchange current to the formation of delayed after depolarizations in intact rat ventricular muscle. J Cardiovasc Pharmacol 53:517–522. https://doi.org/10.1097/FJC.0b013e3181a913f4
Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S (2008) Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103:1466–1472. https://doi.org/10.1161/CIRCRESAHA.108.184457
ter Keurs HEDJ, Boyden PA (2007) Calcium and arrhythmogenesis. Physiol Rev 87:457–506
Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS (2011) Reactive oxygen species-activated ca/calmodulin kinase IIδ is required for late INa augmentation leading to cellular Na and Ca overload. Circ Res 108:555–565. https://doi.org/10.1161/CIRCRESAHA.110.221911
Wakayama Y, Miura M, Stuyvers BD, Boyden PA, ter Keurs HEDJ (2005) Spatial nonuniformity of excitation-contraction coupling causes arrhythmogenic Ca2+ waves in rat cardiac muscle. Circ Res 96:1266–1273
Ward CA, Giles WR (1997) Ionic mechanism of the effects of hydrogen peroxide in rat ventricular myocytes. J Physiol 500:631–642
Xie LH, Chen F, Karagueuzian HS, Weiss JN (2009) Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res 104:79–86. https://doi.org/10.1161/CIRCRESAHA.108.183475
Xu L, Eu JP, Meissner G, Stamler JS (1998) Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279:234–237
Yan Y, Wei CL, Zhang WR, Cheng HP, Liu J (2006) Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol Sin 27:821–826. https://doi.org/10.1111/j.1745-7254.2006.00390.x
Young AA, Dokos S, Powell KA, Sturm B, McCulloch AD, Starling RC, McCarthy PM, White RD (2001) Regional heterogeneity of function in nonischemic dilated cardiomyopathy. Cardiovasc Res 49:308–318
Zhang H, Gomez AM, Wang X, Yan Y, Zheng M, Cheng H (2013) ROS regulation of microdomain Ca2+ signalling at the dyads. Cardiovasc Res 98:248–258. https://doi.org/10.1093/cvr/cvt050
Zima AV, Blatter LA (2006) Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 71:310–321
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by Grant-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science (M. Miura, No. 26460288; C Shindoh No. 16K08485).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miura, M., Taguchi, Y., Handoh, T. et al. Regional increase in ROS within stretched region exacerbates arrhythmias in rat trabeculae with nonuniform contraction. Pflugers Arch - Eur J Physiol 470, 1349–1357 (2018). https://doi.org/10.1007/s00424-018-2152-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2152-x