Abstract
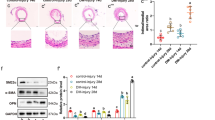
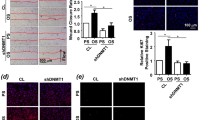
DJ-1 and sphingosine-1-phosphate (S1P) receptors (S1PRs) are implicated in the control of physiology and pathophysiology of cardiovascular systems such as blood pressure, atherosclerosis, and restenosis. Here, we investigated whether DJ-1 with antioxidant function participates in the regulation of S1PR1 and S1PR2 expression in vascular smooth muscle cells (VSMCs) and whether this response is related to vascular neointima formation. In vitro studies used cellular migration assay, western blot, reverse transcriptase and real-time PCR analysis, and immunocytochemistry. In vivo studies were performed using the carotid artery ligation model together with immunohistochemistry in DJ-1 knockout (DJKO) and corresponding wild-type (DJWT) mice. S1P stimulated migration of VSMCs from DJKO and DJWT mice. VSMC migration was suppressed by S1PR1 inhibitor but was elevated by S1PR2 inhibitor. Compared with DJWT mice, S1PR1 expression was higher in VSMCs and neointimal plaque from DJKO mice, but S1PR2 expression was lower. Overexpression of DJ-1 in DJKO VSMCs reduced S1PR1 expression and elevated S1PR2 expression. Compared with DJWT mice, histone deacetylase-1 recruitment and histone H3 acetylation at the S1PR1 promoter region were lower and higher, respectively, but this pattern was reversed at the S1PR2 promoter region in DJKO VSMCs. S1PR expressions and epigenetic changes at S1PR promoter regions in DJWT VSMCs treated with H2O2 showed similar patterns to those in DJKO VSMCs. Our findings suggest that DJ-1 may be involved in the regulation of S1PR1 and S1PR2 expression via H2O2-mediated histone modification in VSMCs. Consequently, this modification may affect S1P-induced VSMC migration and be related to vascular neointima formation.







Similar content being viewed by others
References
Blaho VA, Hla T (2014) An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res 55:1596–1608
Brakch N, Dormond O, Bekri S, Golshayan D, Correvon M, Mazzolai L, Steinmann B, Barbey F (2010) Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Eur Heart J 31:67–76
Byon CH, Heath JM, Chen Y (2016) Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol 9:244–253
Culleton BA, Lall P, Kinsella GK, Doyle S, McCaffrey J, Fitzpatrick DA, Burnell AM (2015) A role for the Parkinson’s disease protein DJ-1 as a chaperone and antioxidant in the anhydrobiotic nematode Panagrolaimus superbus. Cell Stress Chaperones 20:121–137
Duru EA, Fu Y, Davies MG (2012) Role of S-1-P receptors and human vascular smooth muscle cell migration in diabetes and metabolic syndrome. J Surg Res 177:e75–e82
Findeisen HM, Gizard F, Zhao Y, Qing H, Heywood EB, Jones KL, Cohn D, Bruemmer D (2011) Epigenetic regulation of vascular smooth muscle cell proliferation and neointima formation by histone deacetylase inhibition. Arterioscler Thromb Vasc Biol 31:851–860
He YH, Wang XQ, Zhang J, Liu ZH, Pan WQ, Shen Y, Zhu ZB, Wang LJ, Yan XX, Yang K, Zhang RY, Shen WF, Ding FH, Lu L (2017) Association of serum HMGB2 levels with in-stent restenosis: HMGB2 promotes neointimal hyperplasia in mice with femoral artery injury and proliferation and migration of vSMCs. Arterioscler Thromb Vasc Biol 37:717–729
Herranz M, Esteller M (2007) DNA methylation and histone modifications in patients with cancer: potential prognostic and therapeutic targets. Methods Mol Biol 361:25–62
Igarashi J, Miyoshi M, Hashimoto T, Kubota Y, Kosaka H (2007) Hydrogen peroxide induces S1P1 receptors and sensitizes vascular endothelial cells to sphingosine 1-phosphate, a platelet-derived lipid mediator. Am J Physiol Cell Physiol 292:C740–C748
Inoue S, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy M (2007) Regulation of arterial lesions in mice depends on differential smooth muscle cell migration: a role for sphingosine-1-phosphate receptors. J Vasc Surg 46:756–763
Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM (2009) Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res 87:123–129
Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Gräler M, Heusch G, Levkau B (2011) Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ Res 108:314–323
Kim GH, Ryan JJ, Archer SL (2013) The role of redox signaling in epigenetics and cardiovascular disease. Antioxid Redox Signal 18:1920–1936
Lee HM, Lee CK, Lee SH, Roh HY, Bae YM, Lee KY, Lim J, Park PJ, Park TK, Lee YL, Won KJ, Kim B (2007) p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci 105:74–81
Lee KP, Won KJ, Lee DH, Lee DY, Jung SH, Baek S, Park TS, Kim B (2015) DJ-1-mediated upregulation of serine palmitoyltransferase 2 controls vascular neointima via S1P autocrine. Int J Cardiol 191:220–222
Lee DY, Kim HS, Won KJ, Lee KP, Jung SH, Park ES, Choi WS, Lee HM, Kim B (2015) DJ-1 regulates the expression of renal (pro)renin receptor via reactive oxygen species-mediated epigenetic modification. Biochim Biophys Acta 1850:426–434
Machida T, Matamura R, Iizuka K, Hirafuji M (2016) Cellular function and signaling pathways of vascular smooth muscle cells modulated by sphingosine 1-phosphate. J Pharmacol Sci 132:211–217
Madamanchi NR, Runge MS (2013) Redox signaling in cardiovascular health and disease. Free Radic Biol Med 61:473–501
Marx SO, Totary-Jain H, Marks AR (2011) Vascular smooth muscle cell proliferation in restenosis. Circ Cardiovasc Interv 4:104–111
Monks TJ, Xie R, Tikoo K, Lau SS (2006) Ros-induced histone modifications and their role in cell survival and cell death. Drug Metab Rev 38:755–767
Oh SE, Mouradian MM (2018) Cytoprotective mechanisms of DJ-1 against oxidative stress through modulating ERK1/2 and ASK1 signal transduction. Redox Biol 14:211–217
Opsahl JA, Hjørnevik LV, Bull VH, Fismen L, Frøyset AK, Gromyko D, Solstad T, Fladmark KE (2010) Increased interaction between DJ-1 and the Mi-2/nucleosome remodelling and deacetylase complex during cellular stress. Proteomics 10:1494–1504
Porter KE, Riches K (2013) The vascular smooth muscle cell: a therapeutic target in type 2 diabetes? Clin Sci (Lond) 125:167–182
Proia RL, Hla T (2005) Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest 125:1379–1387
Pyne NJ, El Buri A, Adams DR, Pyne S (2012) Sphingosine 1-phosphate signalling in cancer. Biochem Soc Trans 40:94–100
Reich KA, Chen YW, Thompson PD, Hoffman EP, Clarkson PM (2010) Forty-eight hours of unloading and 24 h of reloading lead to changes in global gene expression patterns related to ubiquitination and oxidative stress in humans. J Appl Physiol 109:1404–1415
Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S (2009) Sphingosine 1-phosphate receptor signaling. Annu Rev Biochem 78:743–768
San Martín A, Griendling KK (2010) Redox control of vascular smooth muscle migration. Antioxid Redox Signal 12:625–640
Schuchardt M, Tölle M, Prüfer J, van der Giet M (2011) Pharmacological relevance and potential of sphingosine 1-phosphate in the vascular system. Br J Pharmacol 163:1140–1162
Schwartz SM (1997) Perspectives series: cell adhesion in vascular biology. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest 99:2814–2816
Shimizu T, Nakazawa T, Cho A, Dastvan F, Shilling D, Daum G, Reidy MA (2007) Sphingosine 1-phosphate receptor 2 negatively regulates neointimal formation in mouse arteries. Circ Res 101:995–1000
Shimizu T, De Wispelaere A, Winkler M, D'Souza T, Caylor J, Chen L, Dastvan F, Deou J, Cho A, Larena-Avellaneda A, Reidy M, Daum G (2012) Sphingosine-1-phosphate receptor 3 promotes neointimal hyperplasia in mouse iliac-femoral arteries. Arterioscler Thromb Vasc Biol 32:955–9616
Spiegel S, Milstien S (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 11:403–415
Takashima S, Sugimoto N, Takuwa N, Okamoto Y, Yoshioka K, Takamura M, Takata S, Kaneko S, Takuwa Y (2008) G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on rho but not rho kinase. Cardiovasc Res 79:689–697
Usui T, Morita T, Okada M, Yamawaki H (2014) Histone deacetylase 4 controls neointimal hyperplasia via stimulating proliferation and migration of vascular smooth muscle cells. Hypertension 63:397–403
Wamhoff BR, Lynch KR, Macdonald TL, Owens GK (2008) Sphingosine-1-phosphate receptor subtypes differentially regulate smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 28:1454–1461
Wilson MA (2011) The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal 15:111–122
Won KJ, Jung SH, Lee CK, Na HR, Lee KP, Lee DY, Park ES, Choi WS, Shim SB, Kim B (2013) DJ-1/park7 protects against neointimal formation via the inhibition of vascular smooth muscle cell growth. Cardiovasc Res 97:553–561
Won KJ, Jung SH, Jung SH, Lee KP, Lee HM, Lee DY, Park ES, Kim J, Kim B (2014) DJ-1/park7 modulates vasorelaxation and blood pressure via epigenetic modification of endothelial nitric oxide synthase. Cardiovasc Res 101:473–481
Xu SS, Alam S, Margariti A (2014) Epigenetics in vascular disease—therapeutic potential of new agents. Curr Vasc Pharmacol 12:77–86
Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL (2005) Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet 14:2063–2073
Zhao H, Han Z, Ji X, Luo Y (2016) Epigenetic regulation of oxidative stress in ischemic stroke. Aging Dis 7:295–306
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea, NRF, funded by the Ministry of Education, Science and Technology (NRF 2014R1A2A2A01007329, 2017R1D1A1B03035674) and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HI15C1540).
Author information
Authors and Affiliations
Contributions
K.P.L., B.K., and K.J.W. conceived and designed this research; K.P.L., S.B., S.H.J., D.L., and L.C. performed the acquisition of data; K.P.L., S.B., B.K., H.W.C., and K.J.W. analyzed the data; K.P.L., S.B., L.B.H., B.K., and K.J.W. interpreted the data; K.P.L., S.B., B.K., and K.J.W. drafted the manuscript; and K.P.L., S.B., S.H.J., B.K., and K.J.W. prepared the figures. All the authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
All animal procedures in this study were in strict adherence to the Guide for the Care and Use of Laboratory Animals as adopted by the US National Institutes of Health (NIH publication mo. 85-23, revised 2011) and were approved by the Animal Subjects Committee and by the Institutional Guidelines of Konkuk University, Korea.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 90 kb)
Rights and permissions
About this article
Cite this article
Lee, K.P., Baek, S., Jung, S.H. et al. DJ-1 is involved in epigenetic control of sphingosine-1-phosphate receptor expression in vascular neointima formation. Pflugers Arch - Eur J Physiol 470, 1103–1113 (2018). https://doi.org/10.1007/s00424-018-2132-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2132-1