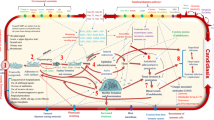
Abstract
The integrity of tight junctions, which regulate paracellular permeability, is challenged by many bacterial pathogens. This is caused by inflammatory responses triggered by pathogens and direct interaction of bacteria or their toxins with host epithelial cells. In some cases, tight junction proteins represent receptors for cell surface proteins or toxins of the pathogen, such as Clostridium perfringens enterotoxin (CPE). CPE causes diarrhea and cramps—the symptoms of a common foodborne illness, caused by C. perfringens type A. It uses a subgroup of the claudin family of tight junction proteins as receptors and forms pores in the membrane of intestinal epithelial cells. Ca2+ influx through these pores finally triggers cell damage. In this review, we summarize tight junction targeting and alteration by a multitude of different microorganisms such as C. perfringens, Escherichia coli, Helicobacter pylori, Salmonella typhimurium, Shigella flexneri, Vibrio cholerae, Yersinia enterocolitica, protozoan parasites, and their proteins. A focus is drawn towards CPE, the interaction with its receptors, cellular, and pathophysiological consequences for the intestinal epithelium. In addition, we portend to the use of CPE-based claudin modulators for drug delivery as well as diagnosis and therapy of cancer.


Similar content being viewed by others
References
Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S (2003) Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430–1434. doi:10.1126/science.1081919
Autheman D, Wyder M, Popoff M, D’Herde K, Christen S, Posthaus H (2013) Clostridium perfringens beta-toxin induces necrostatin-inhibitable, calpain-dependent necrosis in primary porcine endothelial cells. PLoS One 8:e64644. doi:10.1371/journal.pone.0064644
Awad MM, Bryant AE, Stevens DL, Rood JI (1995) Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol Microbiol 15:191–202. doi:10.1111/j.1365-2958.1995.tb02234.x
Backert S, Clyne M, Tegtmeyer N (2011) Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal 9:28. doi:10.1186/1478-811X-9-28
Ben-David U, Nudel N, Benvenisty N (2013) Immunologic and chemical targeting of the tight-junction protein claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat Commun 4:1992. doi:10.1038/ncomms2992
Bertelsen LS, Paesold G, Marcus SL, Finlay BB, Eckmann L, Barrett KE (2004) Modulation of chloride secretory responses and barrier function of intestinal epithelial cells by the Salmonella effector protein SigD. Am J Physiol Cell Physiol 287:C939–C948. doi:10.1152/ajpcell.00413.2003
Bos J, Smithee L, McClane B, Distefano RF, Uzal F, Songer JG, Mallonee S, Crutcher JM (2005) Fatal necrotizing colitis following a foodborne outbreak of enterotoxigenic Clostridium perfringens type A infection. Clin Infect Dis 40:E78–E83. doi:10.1086/429829
Boyle EC, Brown NF, Finlay BB (2006) Salmonella enterica serovar typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell Microbiol 8:1946–1957. doi:10.1111/j.1462-5822.2006.00762.x
Briggs DC, Naylor CE, Smedley JG III, Lukoyanova N, Robertson S, Moss DS, McClane BA, Basak AK (2011) Structure of the food-poisoning Clostridium perfringens enterotoxin reveals similarity to the aerolysin-like pore-forming toxins. J Mol Biol 413(1):138–149. doi:10.1016/j.jmb.2011.07.066
Bücker R, Krug SM, Rosenthal R, Günzel D, Fromm A, Zeitz M, Chakraborty T, Fromm M, Epple HJ, Schulzke JD (2011) Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells. J Infect Dis 204:1283–1292. doi:10.1093/infdis/jir504
Bücker R, Schulz E, Günzel D, Bojarski C, Lee IF, John LJ, Wiegand S, Janssen T, Wieler LH, Dobrindt U, Beutin L, Ewers C, Fromm M, Siegmund B, Troeger H, Schulzke JD (2014) Alpha-haemolysin of Escherichia coli in IBD: a potentiator of inflammatory activity in the colon. Gut 63:1893–1901. doi:10.1136/gutjnl-2013-306099
Caserta JA, Robertson SL, Saputo J, Shrestha A, McClane BA, Uzal FA (2011) Development and application of a mouse intestinal loop model to study the in vivo action of Clostridium perfringens enterotoxin. Infect Immun 79:3020–3027. doi:10.1128/IAI.01342-10
Chakrabarti G, McClane BA (2005) The importance of calcium influx, calpain and calmodulin for the activation of CaCo-2 cell death pathways by Clostridium perfringens enterotoxin. Cell Microbiol 7:129–146. doi:10.1111/j.1462-5822.2004.00442.x
Chakrabarti G, Zhou X, McClane BA (2003) Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect Immun 71:4260–4270. doi:10.1128/IAI.71.8.4260-4270.2003
Chen J, Ma M, Uzal FA, McClane BA (2014) Host cell-induced signaling causes Clostridium perfringens to upregulate production of toxins important for intestinal infections. Gut Microbes 5:96–107. doi:10.4161/gmic.26419
Chen J, Theoret JR, Shrestha A, Smedley JG 3rd, McClane BA (2012) Cysteine-scanning mutagenesis supports the importance of Clostridium perfringens enterotoxin amino acids 80 to 106 for membrane insertion and pore formation. Infect Immun 80:4078–4088. doi:10.1128/IAI.00069-12
Cocco E, Casagrande F, Bellone S, Richter CE, Bellone M, Todeschini P, Holmberg JC, Fu HH, Montagna MK, Mor G, Schwartz PE, Arin-Silasi D, Azoudi M, Rutherford TJ, Abu-Khalaf M, Pecorelli S, Santin AD (2010) Clostridium perfringens enterotoxin carboxy-terminal fragment is a novel tumor-homing peptide for human ovarian cancer. BMC Cancer 10:349. doi:10.1186/1471-2407-10-349
Dean P, Kenny B (2004) Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol Microbiol 54:665–675. doi:10.1111/j.1365-2958.2004.04308.x
Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A (2001) Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem 276:19160–19165. doi:10.1074/jbc.M009674200
Dorca-Arevalo J, Pauillac S, Diaz-Hidalgo L, Martin-Satue M, Popoff MR, Blasi J (2014) Correlation between in vitro cytotoxicity and in vivo lethal activity in mice of epsilon toxin mutants from Clostridium perfringens. PLoS One 9:e102417. doi:10.1371/journal.pone.0102417
Ebihara C, Kondoh M, Harada M, Fujii M, Mizuguchi H, Tsunoda S, Horiguchi Y, Yagi K, Watanabe Y (2007) Role of Tyr306 in the C-terminal fragment of Clostridium perfringens enterotoxin for modulation of tight junction. Biochem Pharmacol 73:824–830. doi:10.1016/j.bcp.2006.11.013
Fasano A, Uzzau S, Fiore C, Margaretten K (1997) The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology 112:839–846. doi:10.1053/gast.1997.v112.pm9041245
Fernandez-Miyakawa ME, Pistone Creydt V, Uzal FA, McClane BA, Ibarra C (2005) Clostridium perfringens enterotoxin damages the human intestine in vitro. Infect Immun 73:8407–8410. doi:10.1128/IAI.73.12.8407-8410.2005
Fisher DJ, Fernandez-Miyakawa ME, Sayeed S, Poon R, Adams V, Rood JI, Uzal FA, McClane BA (2006) Dissecting the contributions of Clostridium perfringens type C toxins to lethality in the mouse intravenous injection model. Infect Immun 74:5200–5210. doi:10.1128/IAI.00534-06
Fujita K, Katahira J, Horiguchi Y, Sonoda N, Furuse M, Tsukita S (2000) Clostridium perfringens enterotoxin binds to the second extracellular loop of claudin-3, a tight junction integral membrane protein. FEBS Lett 476:258–261. doi:10.1016/S0014-5793(00)01744-0
Fukumatsu M, Ogawa M, Arakawa S, Suzuki M, Nakayama K, Shimizu S, Kim M, Mimuro H, Sasakawa C (2012) Shigella targets epithelial tricellular junctions and uses a noncanonical clathrin-dependent endocytic pathway to spread between cells. Cell Host Microbe 11:325–336. doi:10.1016/j.chom.2012.03.001
Garcia JP, Li J, Shrestha A, Freedman JC, Beingesser J, McClane BA, Uzal FA (2014) Clostridium perfringens type A enterotoxin damages the rabbit colon. Infect Immun 82:2211–2218. doi:10.1128/IAI.01659-14
Gerlach RG, Hensel M (2007) Protein secretion systems and adhesins: the molecular armory of gram-negative pathogens. Int J Med Microbiol 297:401–415. doi:10.1016/j.ijmm.2007.03.017
Goldstein J, Morris WE, Loidl CF, Tironi-Farinati C, McClane BA, Uzal FA, Fernandez-Miyakawa ME (2009) Clostridium perfringens epsilon toxin increases the small intestinal permeability in mice and rats. PLoS One 4:e7065. doi:10.1371/journal.pone.0007065
Gui L, Subramony C, Fratkin J, Hughson MD (2002) Fatal enteritis necroticans (pigbel) in a diabetic adult. Mod Pathol 15:66–70. doi:10.1038/modpathol.3880491
Guttman JA, Samji FN, Li Y, Vogl AW, Finlay BB (2006) Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect Immun 74:6075–6084. doi:10.1128/IAI.00721-06
Hanajima-Ozawa M, Matsuzawa T, Fukui A, Kamitani S, Ohnishi H, Abe A, Horiguchi Y, Miyake M (2007) Enteropathogenic Escherichia coli, Shigella flexneri, and Listeria monocytogenes recruit a junctional protein, zonula occludens-1, to actin tails and pedestals. Infect Immun 75:565–573. doi:10.1128/IAI.01479-06
Hanna PC, Mietzner TA, Schoolnik GK, McClane BA (1991) Localization of the receptor-binding region of Clostridium perfringens enterotoxin utilizing cloned toxin fragments and synthetic peptides. The 30 C-terminal amino acids define a functional binding region. J Biol Chem 266:11037–11043
Harada M, Kondoh M, Ebihara C, Takahashi A, Komiya E, Fujii M, Mizuguchi H, Tsunoda S, Horiguchi Y, Yagi K, Watanabe Y (2007) Role of tyrosine residues in modulation of claudin-4 by the C-terminal fragment of Clostridium perfringens enterotoxin. Biochem Pharmacol 73:206–214. doi:10.1016/j.bcp.2006.10.002
Hardy SP, Denmead M, Parekh N, Granum PE (1999) Cationic currents induced by Clostridium perfringens type A enterotoxin in human intestinal CaCo-2 cells. J Med Microbiol 48:235–243. doi:10.1099/00222615-48-3-235
Hatheway CL (1990) Toxigenic clostridia. Clin Microbiol Rev 3:66–98
Hemmasi S, Czulkies BA, Schorch B, Veit A, Aktories K, Papatheodorou P (2015) Interaction of the Clostridium difficile binary toxin CDT and its host cell receptor, lipolysis-stimulated lipoprotein receptor (LSR). J Biol Chem 290:14031–14044. doi:10.1074/jbc.M115.650523
Hering NA, Fromm A, Kikhney J, Lee IF, Moter A, Schulzke JD, Bücker R (2016) Yersinia enterocolitica affects intestinal barrier function in the colon. J Infect Dis 213:1157–1162. doi:10.1093/infdis/jiv571
Hering NA, Richter JF, Krug SM, Günzel D, Fromm A, Bohn E, Rosenthal R, Bücker R, Fromm M, Troeger H, Schulzke JD (2011) Yersinia enterocolitica induces epithelial barrier dysfunction through regional tight junction changes in colonic HT-29/B6 cell monolayers. Lab Investig 91:310–324. doi:10.1038/labinvest.2010.180
Hodges K, Gill R (2010) Infectious diarrhea: cellular and molecular mechanisms. Gut Microbes 1:4–21. doi:10.4161/gmic.1.1.11036
Horiguchi Y, Uemura T, Kozaki S, Sakaguchi G (1986) Effects of Ca2+ and other cations on the action of Clostridium perfringens enterotoxin. Biochim Biophys Acta 889:65–71. doi:10.1016/0167-4889(86)90009-1
Iacovache I, De Carlo S, Cirauqui N, Dal Peraro M, van der Goot FG, Zuber B (2016) Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat Commun 7:12062. doi:10.1038/ncomms12062
Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S (2005) Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171:939–945. doi:10.1083/jcb.200510043
John LJ, Fromm M, Schulzke JD (2011) Epithelial barriers in intestinal inflammation. Antioxid Redox Signal 15:1255–1270. doi:10.1089/ars.2011.3892
Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N (1997) Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol 136:1239–1247
Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N (1997) Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem 272:26652–26658. doi:10.1074/jbc.272.42.26652
Keyburn AL, Boyce JD, Vaz P, Bannam TL, Ford ME, Parker D, Di Rubbo A, Rood JI, Moore RJ (2008) NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog 4:e26. doi:10.1371/journal.ppat.0040026
Kimura J, Abe H, Kamitani S, Toshima H, Fukui A, Miyake M, Kamata Y, Sugita-Konishi Y, Yamamoto S, Horiguchi Y (2010) Clostridium perfringens enterotoxin interacts with claudins via electrostatic attraction. J BiolChem 285:401–408. doi:10.1074/jbc.M109.051417
Kitadokoro K, Nishimura K, Kamitani S, Fukui-Miyazaki A, Toshima H, Abe H, Kamata Y, Sugita-Konishi Y, Yamamoto S, Karatani H, Horiguchi Y (2011) Crystal structure of Clostridium perfringens enterotoxin displays features of beta-pore-forming toxins. J Biol Chem 286:19549–19555. doi:10.1074/jbc.M111.228478
Kobelt D, Aumann J, Schmidt M, Wittig B, Fichtner I, Behrens D, Lemm M, Freundt G, Schlag PM, Walther W (2014) Preclinical study on combined chemo- and nonviral gene therapy for sensitization of melanoma using a human TNF-alpha expressing MIDGE DNA vector. Mol Oncol 8:609–619. doi:10.1016/j.molonc.2013.12.019
Kohler H, Sakaguchi T, Hurley BP, Kase BA, Reinecker HC, McCormick BA (2007) Salmonella enterica serovar typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol 293:G178–G187. doi:10.1152/ajpgi.00535.2006
Kokai-Kun JF, McClane BA (1997) Deletion analysis of the Clostridium perfringens enterotoxin. Infect Immun 65:1014–1022
Kominsky SL, Tyler B, Sosnowski J, Brady K, Doucet M, Nell D, Smedley JG III, McClane B, Brem H, Sukumar S (2007) Clostridium perfringens enterotoxin as a novel-targeted therapeutic for brain metastasis. Cancer Res 67:7977–7982. doi:10.1158/0008-5472.CAN-07-1314
Kominsky SL, Vali M, Korz D, Gabig TG, Weitzman SA, Argani P, Sukumar S (2004) Clostridium perfringens enterotoxin elicits rapid and specific cytolysis of breast carcinoma cells mediated through tight junction proteins claudin 3 and 4. Am J Pathol 164:1627–1633. doi:10.1016/S0002-9440(10)63721-2
Kondoh M, Takahashi A, Fujii M, Yagi K, Watanabe Y (2006) A novel strategy for a drug delivery system using a claudin modulator. Biol Pharm Bull 29:1783–1789. doi:10.1248/bpb.29.1783
Krause G, Protze J, Piontek J (2015) Assembly and function of claudins: structure-function relationships based on homology models and crystal structures. Semin Cell Dev Biol 42:3–12. doi:10.1016/j.semcdb.2015.04.010
Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins. Biochim Biophys Acta 1778:631–645. doi:10.1016/j.bbamem.2007.10.018
Krueger S, Hundertmark T, Kuester D, Kalinski T, Peitz U, Roessner A (2007) Helicobacter pylori alters the distribution of ZO-1 and p120ctn in primary human gastric epithelial cells. Pathol Res Pract 203:433–444. doi:10.1016/j.prp.2007.04.003
Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M (2009) Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol Biol Cell 20:3713–3724. doi:10.1091/mbc.E09-01-0080
Kuehne SA, Collery MM, Kelly ML, Cartman ST, Cockayne A, Minton NP (2014) Importance of toxin a, toxin B, and CDT in virulence of an epidemic Clostridium difficile strain. J Infect Dis 209:83–86. doi:10.1093/infdis/jit426
Loffler A, Labbe R (1986) Characterization of a parasporal inclusion body from sporulating, enterotoxin-positive Clostridium perfringens type A. J Bacteriol 165:542–548
Los FC, Randis TM, Aroian RV, Ratner AJ (2013) Role of pore-forming toxins in bacterial infectious diseases. Microbiol Molecular Biol Rev 77:173–207. doi:10.1128/MMBR.00052-12
Ma T, Verkman AS (1999) Aquaporin water channels in gastrointestinal physiology. J Physiol 517(Pt 2):317–326. doi:10.1111/j.1469-7793.1999.0317t.x
Mahendran V, Liu F, Riordan SM, Grimm MC, Tanaka MM, Zhang L (2016) Examination of the effects of Campylobacter concisus zonula occludens toxin on intestinal epithelial cells and macrophages. Gut Pathog 8:18. doi:10.1186/s13099-016-0101-9
Mancheno JM, Tateno H, Sher D, Goldstein IJ (2010) Laetiporus sulphureus lectin and aerolysin protein family. Adv Exp Med Biol 677:67–80
Masuda S, Oda Y, Sasaki H, Ikenouchi J, Higashi T, Akashi M, Nishi E, Furuse M (2011) LSR defines cell corners for tricellular tight junction formation in epithelial cells. J Cell Sci 124:548–555. doi:10.1242/jcs.072058
Matsuda M, Ozutsumi K, Sugimoto N, Iwahashi H (1986) Primary action of Clostridium perfringens type A enterotoxin on HeLa and Vero cells in the absence of extracellular calcium: rapid and characteristic changes in membrane permeability. Biochem Biophys Res Commun 141(2):704–710
Matsuda M, Sugimoto N (1979) Calcium-independent and dependent steps in action of Clostridium perfringens enterotoxin on Hela and Vero cells. Biochem and Biophys Res Commun 91:629–636. doi:10.1016/0006-291X(79)91568-7
Matsuda T, Okada Y, Inagi E, Tanabe Y, Shimizu Y, Nagashima K, Sakurai J, Nagahama M, Tanaka S (2007) Enteritis necroticans ‘pigbel’ in a Japanese diabetic adult. Pathol Int 57:622–626. doi:10.1111/j.1440-1827.2007.02149.x
Matsuzawa T, Kuwae A, Abe A (2005) Enteropathogenic Escherichia coli type III effectors EspG and EspG2 alter epithelial paracellular permeability. Infect Immun 73:6283–6289. doi:10.1128/IAI.73.10.6283-6289.2005
McDonel JL (1980) Clostridium perfringens toxins (type A, B, C, D, E). Pharmacol Ther 10:617–655. doi:10.1016/0163-7258(80)90031-5
McDonel JL, Demers GW (1982) In vivo effects of enterotoxin from Clostridium perfringens type A in the rabbit colon: binding vs. biologic activity. J Infect Dis 145:490–494. doi:10.1093/infdis/145.4.490
McDonel JL (1986) Toxins of Clostridium perfringens types a, B, C, D and E. In: Dorner F, Drews H (eds) Pharmacology of bacterial toxins. Pergamon Press, Oxford, pp. 477–517
Michl P, Buchholz M, Rolke M, Kunsch S, Lohr M, McClane B, Tsukita S, Leder G, Adler G, Gress TM (2001) Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology 121:678–684. doi:10.1053/gast.2001.27124
Mogk S, Meiwes A, Shtopel S, Schraermeyer U, Lazarus M, Kubata B, Wolburg H, Duszenko M (2014) Cyclical appearance of African trypanosomes in the cerebrospinal fluid: new insights in how trypanosomes enter the CNS. PLoS One 9:e91372. doi:10.1371/journal.pone.0091372
Monturiol-Gross L, Flores-Diaz M, Campos-Rodriguez D, Mora R, Rodriguez-Vega M, Marks DL, Alape-Giron A (2014) Internalization of Clostridium perfringens alpha-toxin leads to ERK activation and is involved on its cytotoxic effect. Cell Microbiol 16:535–547. doi:10.1111/cmi.12237
Morin PJ (2007) Claudin proteins in ovarian cancer. Dis Markers 23:453–457. doi:10.1155/2007/674058
Morita K, Furuse M, Fujimoto K, Tsukita S (1999) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A 96:511–516
Mosley M, Knight J, Neesse A, Michl P, Iezzi M, Kersemans V, Cornelissen B (2015) Claudin-4 SPECT imaging allows detection of aplastic lesions in a mouse model of breast cancer. J Nucl Med 56:745–751. doi:10.2967/jnumed.114.152496
Muza-Moons MM, Schneeberger EE, Hecht GA (2004) Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol 6:783–793. doi:10.1111/j.1462-5822.2004.00404.x
Nagahama M, Hayashi S, Morimitsu S, Sakurai J (2003) Biological activities and pore formation of Clostridium perfringens beta toxin in HL 60 cells. J Biol Chem 278:36934–36941. doi:10.1074/jbc.M306562200
Nagahama M, Sakurai J (1991) Distribution of labeled Clostridium perfringens epsilon toxin in mice. Toxicon 29:211–217
Nava P, Vidal JE (2016) The CpAL system regulates changes of the trans-epithelial resistance of human enterocytes during Clostridium perfringens type C infection. Anaerobe 39:143–149. doi:10.1016/j.anaerobe.2016.04.002
Neesse A, Hahnenkamp A, Griesmann H, Buchholz M, Hahn SA, Maghnouj A, Fendrich V, Ring J, Sipos B, Tuveson DA, Bremer C, Gress TM, Michl P (2013) Claudin-4-targeted optical imaging detects pancreatic cancer and its precursor lesions. Gut 62:1034–1043. doi:10.1136/gutjnl-2012-302577
Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA (2001) Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun 69:1329–1336. doi:10.1128/IAI.69.3.1329-1336.2001
Oda M, Terao Y, Sakurai J, Nagahama M (2015) Membrane-binding mechanism of Clostridium perfringens alpha-toxin. Toxins (Basel) 7:5268–5275. doi:10.3390/toxins7124880
Olsen SJ, MacKinnon LC, Goulding JS, Bean NH, Slutsker L (2000) Surveillance for foodborne-disease outbreaks—United States, 1993–1997. MMWR CDC Surveill Summ 49:1–62
Papatheodorou P, Carette JE, Bell GW, Schwan C, Guttenberg G, Brummelkamp TR, Aktories K (2011) Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc Natl Acad Sci U S A 108:16422–16427. doi:10.1073/pnas.1109772108
Peralta-Ramirez J, Hernandez JM, Manning-Cela R, Luna-Munoz J, Garcia-Tovar C, Nougayrede JP, Oswald E, Navarro-Garcia F (2008) EspF interacts with nucleation-promoting factors to recruit junctional proteins into pedestals for pedestal maturation and disruption of paracellular permeability. Infect Immun 76:3854–3868. doi:10.1128/IAI.00072-08
Petit L, Gibert M, Gourch A, Bens M, Vandewalle A, Popoff MR (2003) Clostridium perfringens epsilon toxin rapidly decreases membrane barrier permeability of polarized MDCK cells. Cell Microbiol 5:155–164. doi:10.1046/j.1462-5822.2003.00262.x
Petrillo TM, Beck-Sague CM, Songer JG, Abramowsky C, Fortenberry JD, Meacham L, Dean AG, Lee H, Bueschel DM, Nesheim SR (2000) Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med 342:1250–1253. doi:10.1056/NEJM200004273421704
Protze J, Eichner M, Piontek A, Dinter S, Rossa J, Blecharz KG, Vajkoczy P, Piontek J, Krause G (2015) Directed structural modification of Clostridium perfringens enterotoxin to enhance binding to claudin-5. Cell Mol Life Sci 72:1417–1432. doi:10.1007/s00018-014-1761-6
Rajabian T, Gavicherla B, Heisig M, Muller-Altrock S, Goebel W, Gray-Owen SD, Ireton K (2009) The bacterial virulence factor InlC perturbs apical cell junctions and promotes cell-to-cell spread of Listeria. Nat Cell Biol 11:1212–1218. doi:10.1038/ncb1964
Richard JF, Mainguy G, Gibert M, Marvaud JC, Stiles BG, Popoff MR (2002) Transcytosis of iota-toxin across polarized CaCo-2 cells. Mol Microbiol 43:907–917. doi:10.1046/j.1365-2958.2002.02806.x
Robertson SL, Smedley JG III, Singh U, Chakrabarti G, Van Itallie CM, Anderson JM, McClane BA (2007) Compositional and stoichiometric analysis of Clostridium perfringens enterotoxin complexes in Caco-2 cells and claudin 4 fibroblast transfectants. Cell Microbiol 9:2734–2755. doi:10.1111/j.1462-5822.2007.00994.x
Robertson SL, Smedley JG, McClane BA (2010) Identification of a claudin-4 residue important for mediating the host cell binding and action of Clostridium perfringens enterotoxin. Inf ect Immun 78:505–517. doi:10.1128/IAI.00778-09
Romanov V, Whyard TC, Waltzer WC, Gabig TG (2014) A claudin 3 and claudin 4-targeted Clostridium perfringens protoxin is selectively cytotoxic to PSA-producing prostate cancer cells. Cancer Lett 351:260–264. doi:10.1016/j.canlet.2014.06.009
Saadat I, Higashi H, Obuse C, Umeda M, Murata-Kamiya N, Saito Y, Lu H, Ohnishi N, Azuma T, Suzuki A, Ohno S, Hatakeyama M (2007) Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature 447:330–333. doi:10.1038/nature05765
Saeki R, Kondoh M, Kakutani H, Matsuhisa K, Takahashi A, Suzuki H, Kakamu Y, Watari A, Yagi K (2010) A claudin-targeting molecule as an inhibitor of tumor metastasis. J Pharmacol Exp Ther 334:576–582. doi:10.1124/jpet.110.168070
Saitoh Y, Suzuki H, Tani K, Nishikawa K, Irie K, Ogura Y, Tamura A, Tsukita S, Fujiyoshi Y (2015) Tight junctions. Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin. Science 347:775–778. doi:10.1126/science.1261833
Sakaguchi T, Kohler H, Gu X, McCormick BA, Reinecker HC (2002) Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol 4:367–381. doi:10.1046/j.1462-5822.2002.00197.x
Sakurai J, Nagahama M, Oda M, Tsuge H, Kobayashi K (2009) Clostridium perfringens iota-toxin: structure and function. Toxins (Basel) 1:208–228. doi:10.3390/toxins1020208
Santin AD, Bellone S, Siegel ER, McKenney JK, Thomas M, Roman JJ, Burnett A, Tognon G, Bandiera E, Pecorelli S (2007) Overexpression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in uterine carcinosarcomas. Clin Cancer Res 13:3339–3346
Santin AD, Cane S, Bellone S, Palmieri M, Siegel ER, Thomas M, Roman JJ, Burnett A, Cannon MJ, Pecorelli S (2005) Treatment of chemotherapy-resistant human ovarian cancer xenografts in C.B-17/SCID mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer Res 65:4334–4342. doi:10.1158/0008-5472.CAN-04-3472
Sarker MR, Carman RJ, McClane BA (1999) Inactivation of the gene (cpe) encoding Clostridium perfringens enterotoxin eliminates the ability of two cpe-positive C. perfringens type A human gastrointestinal disease isolates to affect rabbit ileal loops. Mol Microbiol 33:946–958. doi:10.1046/j.1365-2958.1999.01534.x
Sayeed S, Fernandez-Miyakawa ME, Fisher DJ, Adams V, Poon R, Rood JI, Uzal FA, McClane BA (2005) Epsilon-toxin is required for most Clostridium perfringens type D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect Immun 73:7413–7421. doi:10.1128/IAI.73.11.7413-7421.2005
Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA (2008) Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol Microbiol 67:15–30. doi:10.1111/j.1365-2958.2007.06007.x
Schmidt E, Kelly SM, van der Walle CF (2007) Tight junction modulation and biochemical characterisation of the zonula occludens toxin C-and N-termini. FEBS Lett 581:2974–2980. doi:10.1016/j.febslet.2007.05.051
Schmitz H, Barmeyer C, Gitter AH, Wullstein F, Bentzel CJ, Fromm M, Riecken EO, Schulzke JD (2000) Epithelial barrier and transport function of the colon in ulcerative colitis. Ann N Y Acad Sci 915:312–326. doi:10.1111/j.1749-6632.2000.tb05259.x
Sherman S, Klein E, McClane BA (1994) Clostridium perfringens type A enterotoxin induces tissue damage and fluid accumulation in rabbit ileum. J Diarrhoeal Dis Res 12:200–207
Shinoda T, Shinya N, Ito K, Ohsawa N, Terada T, Hirata K, Kawano Y, Yamamoto M, Kimura-Someya T, Yokoyama S, Shirouzu M (2016) Structural basis for disruption of claudin assembly in tight junctions by an enterotoxin. Sci Rep 6:33632. doi:10.1038/srep33632
Shrestha A, McClane BA (2013) Human claudin-8 and -14 are receptors capable of conveying the cytotoxic effects of Clostridium perfringens enterotoxin. MBio 4(1):e00594–e00512. doi:10.1128/mBio.00594-12
Sidik SM, Huet D, Ganesan SM, Huynh MH, Wang T, Nasamu AS, Thiru P, Saeij JP, Carruthers VB, Niles JC, Lourido S (2016) A genome-wide CRISPR screen in toxoplasma identifies essential apicomplexan genes. Cell 166(1423–1435):e1412. doi:10.1016/j.cell.2016.08.019
Simonovic I, Rosenberg J, Koutsouris A, Hecht G (2000) Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol 2:305–315. doi:10.1046/j.1462-5822.2000.00055.x
Singh U, Mitic LL, Wieckowski EU, Anderson JM, McClane BA (2001) Comparative biochemical and immunocytochemical studies reveal differences in the effects of Clostridium perfringens enterotoxin on polarized CaCo-2 cells versus Vero cells. J Biol Chem 276:33402–33412. doi:10.1074/jbc.M104200200
Singh U, Van Itallie CM, Mitic LL, Anderson JM, McClane BA (2000) CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin. J Biol Chem 275:18407–18417. doi:10.1074/jbc.M001530200
Smedley JG, Saputo J, Parker JC, Fernandez-Miyakawa ME, Robertson SL, McClane BA, Uzal FA (2008) Noncytotoxic Clostridium perfringens enterotoxin (CPE) variants localize CPE intestinal binding and demonstrate a relationship between CPE-induced cytotoxicity and enterotoxicity. Infect Immun 76:3793–3800. doi:10.1128/IAI.00460-08
Smedley JG, Uzal FA, McClane BA (2007) Identification of a prepore large-complex stage in the mechanism of action of Clostridium perfringens enterotoxin. Infect Immun 75:2381–2390. doi:10.1128/IAI.01737-06
Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi Y, Tsukita S (1999) Clostridium perfringens Enterotoxin fragment removes specific claudins from tight junction strands: evidence for direct involvement of claudins in tight junction barrier. J Cell Biol 147:195–204
Suzuki H, Nishizawa T, Tani K, Yamazaki Y, Tamura A, Ishitani R, Dohmae N, Tsukita S, Nureki O, Fujiyoshi Y (2014) Crystal structure of a claudin provides insight into the architecture of tight junctions. Science 344:304–307. doi:10.1126/science.1248571
Szczesny P, Iacovache I, Muszewska A, Ginalski K, van der Goot FG, Grynberg M (2011) Extending the aerolysin family: from bacteria to vertebrates. PLoS One 6:e20349. doi:10.1371/journal.pone.0020349
Takagishi T, Oda M, Kabura M, Kurosawa M, Tominaga K, Urano S, Ueda Y, Kobayashi K, Kobayashi T, Sakurai J, Terao Y, Nagahama M (2015) Clostridium perfringens alpha-toxin induces Gm1a clustering and Trka phosphorylation in the host cell membrane. PLoS One 10:e0120497. doi:10.1371/journal.pone.0120497
Takahashi A, Komiya E, Kakutani H, Yoshida T, Fujii M, Horiguchi Y, Mizuquchi H, Tsutsumi Y, Tsunoda SI, Koizumi N, Isoda K, Yagi K, Watanabe Y, Kondoh M (2008) Domain mapping of a claudin-4 modulator, the C-terminal region of C-terminal fragment of Clostridium perfringens enterotoxin, by site-directed mutagenesis. Biochem Pharmacol 75:1639–1648
Takahashi A, Kondoh M, Suzuki H, Yagi K (2011) Claudin as a target for drug development. Curr Med Chem 18:1861–1865. doi:10.1016/j.bcp.2007.12.016
Takahashi A, Saito Y, Kondoh M, Matsushita K, Krug SM, Suzuki H, Tsujino H, Li X, Aoyama H, Matsuhisa K, Uno T, Fromm M, Hamakubo T, Yagi K (2012) Creation and biochemical analysis of a broad-specific claudin binder. Biomaterials 33:3464–3474. doi:10.1016/j.biomaterials.2012.01.017
Thanabalasuriar A, Kim J, Gruenheid S (2013) The inhibition of COPII trafficking is important for intestinal epithelial tight junction disruption during enteropathogenic Escherichia coli and Citrobacter rodentium infection. Microbes Infect 15:738–744. doi:10.1016/j.micinf.2013.05.001
Thiagarajah JR, Donowitz M, Verkman AS (2015) Secretory diarrhoea: mechanisms and emerging therapies. Nat Rev Gastroenterol Hepatol 12:446–457. doi:10.1038/nrgastro.2015.111
Thiagarajah JR, Verkman AS (2013) Chloride channel-targeted therapy for secretory diarrheas. Curr Opin Pharmacol 13:888–894. doi:10.1016/j.coph.2013.08.005
Urbina P, Collado MI, Alonso A, Goni FM, Flores-Diaz M, Alape-Giron A, Ruysschaert JM, Lensink MF (2011) Unexpected wide substrate specificity of C. perfringens alpha-toxin phospholipase C. Biochim Biophys Acta 1808:2618–2627. doi:10.1016/j.bbamem.2011.06.008
Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA (2014) Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol 9:361–377. doi:10.2217/fmb.13.168
Uzal FA, Saputo J, Sayeed S, Vidal JE, Fisher DJ, Poon R, Adams V, Fernandez-Miyakawa ME, Rood JI, McClane BA (2009) Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C enterotoxemias. Infect Immun 77:5291–5299. doi:10.1128/IAI.00825-09
Uzzau S, Cappuccinelli P, Fasano A (1999) Expression of Vibrio cholerae zonula occludens toxin and analysis of its subcellular localization. Microb Pathog 27:377–385. doi:10.1006/mpat.1999.0312
Van Itallie CM, Betts L, Smedley JG, McClane BA, Anderson JM (2008) Structure of the claudin-binding domain of Clostridium perfringens enterotoxin. J Biol Chem 283:268–274. doi:10.1074/jbc.M708066200
Veshnyakova A, Piontek J, Protze J, Waziri N, Heise I, Krause G (2012) Mechanism of Clostridium perfringens enterotoxin interaction with claudin-3/-4 protein suggests structural modifications of the toxin to target specific claudins. J Biol Chem 287:1698–1708
Veshnyakova A, Protze J, Rossa J, Blasig I, Krause G, Piontek J (2010) On the interaction of Clostridium perfringens enterotoxin with claudins. Toxins 2:1336–1356. doi:10.1074/jbc.M111.312165
Vidal JE, McClane BA, Saputo J, Parker J, Uzal FA (2008) Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon. Infect Immun 76:4396–4404. doi:10.1128/IAI.00547-08
Viswanathan VK, Koutsouris A, Lukic S, Pilkinton M, Simonovic I, Simonovic M, Hecht G (2004) Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect Immun 72:3218–3227. doi:10.1128/IAI.72.6.3218-3227.2004
Walther W, Petkov S, Kuvardina ON, Aumann J, Kobelt D, Fichtner I, Lemm M, Piontek J, Blasig IE, Stein U, Schlag PM (2012) Novel Clostridium perfringens enterotoxin suicide gene therapy for selective treatment of claudin-3- and -4-overexpressing tumors. Gene Ther 19:494–503. doi:10.1038/gt.2011.136
Winkler L, Gehring C, Wenzel A, Muller SL, Piehl C, Krause G, Blasig IE, Piontek J (2009) Molecular determinants of the interaction between Clostridium perfringens enterotoxin fragments and claudin-3. J Biol Chem 284:18863–18872. doi:10.1074/jbc.M109.008623
Wu Z, Nybom P, Magnusson KE (2000) Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol 2:11–17. doi:10.1046/j.1462-5822.2000.00025.x
Yamahashi Y, Saito Y, Murata-Kamiya N, Hatakeyama M (2011) Polarity-regulating kinase partitioning-defective 1b (PAR1b) phosphorylates guanine nucleotide exchange factor H1 (GEF-H1) to regulate RhoA-dependent actin cytoskeletal reorganization. J Biol Chem 286:44576–44584. doi:10.1074/jbc.M111.267021
Yelland TS, Naylor CE, Bagoban T, Savva CG, Moss DS, McClane BA, Blasig IE, Popoff M, Basak AK (2014) Structure of a C. perfringens enterotoxin mutant in complex with a modified claudin-2 extracellular loop 2. J Mol Biol 426:3134–3147. doi:10.1016/j.jmb.2014.07.001
Yuan XQ, Lin XJ, Manorek G, Kanatani I, Cheung LH, Rosenblum MG, Howell SB (2009) Recombinant CPE fused to tumor necrosis factor targets human ovarian cancer cells expressing the claudin-3 and claudin-4 receptors. Mol Cancer Ther 8:1906–1915. doi:10.1158/1535-7163.MCT-09-0106
Zhang J, Ni C, Yang Z, Piontek A, Chen H, Wang S, Fan Y, Qin Z, Piontek J (2015) Specific binding of Clostridium perfringens enterotoxin fragment to claudin-b and modulation of zebrafish epidermal barrier. Exp Dermatol 24:605–610. doi:10.1111/exd.12728
Acknowledgements
The authors gratefully acknowledge support by Sonnenfeld-Stiftung, Berlin; Wilhelm Sander-Stiftung, Munich; and Deutsche Forschungsgemeinschaft PI837/4-1 and KR1273/8-1.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is published as part of the Special Issue on Claudins
Rights and permissions
About this article
Cite this article
Eichner, M., Protze, J., Piontek, A. et al. Targeting and alteration of tight junctions by bacteria and their virulence factors such as Clostridium perfringens enterotoxin. Pflugers Arch - Eur J Physiol 469, 77–90 (2017). https://doi.org/10.1007/s00424-016-1902-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1902-x