Abstract
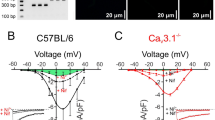
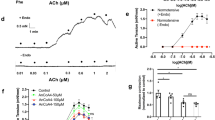
Voltage-gated calcium channels are involved in the vascular excitation-contraction mechanism and regulation of arterial blood pressure. It was hypothesized that T-type channels promote formation of nitric oxide from the endothelium. The present experiments determine the involvement of T-type channels in depolarization-dependent dilatation of mesenteric arteries and blood pressure regulation in Cav3.1 knock-out mice. Nitric oxide-dependent vasodilatation following depolarization-mediated vasoconstriction was reduced significantly in mesenteric arteries from Cav3.1−/− compared to wild type mice. Four days of systemic infusion of a nitric oxide (NO)-synthase-inhibitor to conscious wild type elicited a significant increase in mean arterial blood pressure that was absent in Cav3.1−/− mice. Immunoprecipitation and immunofluorescence labeling showed co-localization of Cav3.1 and endothelial nitric oxide synthase (eNOS) in arteries from wild type mice. Nitric oxide release measured as DAF fluorescence and cGMP levels were significantly lower in depolarized Cav3.1−/− compared to wild type arteries. In summary, the absence of T-type Cav3.1 channels attenuates NO-dependent dilatation in mesenteric arteries in vitro, as well as the hypertension after L-NAME infusion in vivo. Furthermore, Cav3.1 channels cluster with eNOS and promote formation of nitric oxide by the endothelium. The present findings suggest that this mechanism is important for the systemic impact of NO on peripheral resistance.





Similar content being viewed by others
References
Abd El-Rahman RR, Harraz OF, Brett SE, Anfinogenova Y, Mufti RE, Goldman D, Welsh DG (2012) Identification of L- and T-type Ca2+ channels in rat cerebral arteries: role in myogenic tone development. Am J Physiol Heart Circ Physiol 304(1):H58–H71. doi:10.1152/ajpheart.00476.2012
Andersen H, Jaff MG, Hogh D, Vanhoutte P, Hansen PB (2011) Adenosine elicits an eNOS-independent reduction in arterial blood pressure in conscious mice that involves adenosine A2A receptors. Acta Physiol (Oxf) 203(1):197–207
Ball CJ, Wilson DP, Turner SP, Saint DA, Beltrame JF (2009) Heterogeneity of L- and T-channels in the vasculature: rationale for the efficacy of combined L- and T-blockade. Hypertension 53(4):654–660
Bjorling K, Morita H, Olsen MF, Prodan A, Hansen PB, Lory P, Holstein-Rathlou NH, Jensen LJ (2013) Myogenic tone is impaired at low arterial pressure in mice deficient in the low-voltage-activated Ca(V) 3.1 T-type Ca(2+) channel. Acta Physiol (Oxf)
Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ (2008) The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res 46(2):138–151
Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP (2003) Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 302(5649):1416–1418
Figueroa XF, Chen CC, Campbell KP, Damon DN, Day KH, Ramos S, Duling BR (2007) Are voltage-dependent ion channels involved in the endothelial cell control of vasomotor tone? Am J Physiol Heart Circ Physiol 293(3):H1371–H1383. doi:10.1152/ajpheart.01368.2006
Hansen PB (2013) Functional and pharmacological consequences of the distribution of voltage-gated calcium channels in the renal blood vessels. Acta Physiol (Oxf) 207(4):690–699
Hansen PB, Hristovska A, Wolff H, Vanhoutte P, Jensen BL, Bie P (2010) Uridine adenosine tetraphosphate affects contractility of mouse aorta and decreases blood pressure in conscious rats and mice. Acta Physiol (Oxf) 200(2):171–179
Hansen PB, Jensen BL, Andreasen D, Skott O (2001) Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 89(7):630–638
Hansen PB, Poulsen CB, Walter S, Marcussen N, Cribbs LL, Skott O, Jensen BL (2011) Functional importance of L- and p/q-type voltage-gated calcium channels in human renal vasculature. Hypertension 58 (3):464–470
Harraz OF, Brett SE, Welsh DG (2014) Nitric oxide suppresses vascular voltage-gated T-type Ca2+ channels through cGMP/PKG signaling. Am J Physiol Heart Circ Physiol 306(2):H279–H285. doi:10.1152/ajpheart.00743.2013
Hayashi K, Kumagai H, Saruta T (2003) Effect of efonidipine and ACE inhibitors on proteinuria in human hypertension with renal impairment. Am J Hypertens 16(2):116–122
Howitt L, Kuo IY, Ellis A, Chaston DJ, Shin HS, Hansen PB, Hill CE (2013) Chronic deficit in nitric oxide elicits oxidative stress and augments T-type calcium channel contribution to vascular tone of rodent arteries and arterioles. Cardiovasc Res
Isakson BE, Ramos SI, Duling BR (2007) Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100(2):246–254
Kuo IY, Ellis A, Seymour VA, Sandow SL, Hill CE (2010) Dihydropyridine-insensitive calcium currents contribute to function of small cerebral arteries. J Cereb Blood Flow Metab 30(6):1226–1239. doi:10.1038/jcbfm.2010.11
Kuo IY, Wolfle SE, Hill CE (2011) T-type calcium channels and vascular function: the new kid on the block? J Physiol 589(Pt 4):783–795
Lee J, Kim D, Shin HS (2004) Lack of delta waves and sleep disturbances during non-rapid eye movement sleep in mice lacking alpha1G-subunit of T-type calcium channels. Proc Natl Acad Sci U S A 101(52):18195–18199. doi:10.1073/pnas.0408089101
Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P (2006) Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/alpha1G T-type calcium channels. Circ Res 98(11):1422–1430
Nakayama H, Bodi I, Correll RN, Chen X, Lorenz J, Houser SR, Robbins J, Schwartz A, Molkentin JD (2009) alpha1G-dependent T-type Ca2+ current antagonizes cardiac hypertrophy through a NOS3-dependent mechanism in mice. J Clin Invest 119(12):3787–3796
Navarro-Gonzalez MF, Grayson TH, Meaney KR, Cribbs LL, Hill CE (2009) Non-L-type voltage-dependent calcium channels control vascular tone of the rat basilar artery. Clin Exp Pharmacol Physiol 36(1):55–66
Ohishi M, Takagi T, Ito N, Terai M, Tatara Y, Hayashi N, Shiota A, Katsuya T, Rakugi H, Ogihara T (2007) Renal-protective effect of T-and L-type calcium channel blockers in hypertensive patients: an Amlodipine-to-Benidipine Changeover (ABC) study. Hypertens Res 30(9):797–806
Ohta M, Sugawara S, Sato N, Kuriyama C, Hoshino C, Kikuchi A (2009) Effects of benidipine, a long-acting T-type calcium channel blocker, on home blood pressure and renal function in patients with essential hypertension: a retrospective, ‘real-world’ comparison with amlodipine. Clin Drug Investig 29(11):739–746
Poulsen CB, Al-Mashhadi RH, Cribbs LL, Skott O, Hansen PB (2011) T-type voltage-gated calcium channels regulate the tone of mouse efferent arterioles. Kidney Int 79(4):443–451. doi:10.1038/ki.2010.429
Sasaki H, Saiki A, Endo K, Ban N, Yamaguchi T, Kawana H, Nagayama D, Ohhira M, Oyama T, Miyashita Y, Shirai K (2009) Protective effects of efonidipine, a T- and L-type calcium channel blocker, on renal function and arterial stiffness in type 2 diabetic patients with hypertension and nephropathy. J Atheroscler Thromb 16(5):568–575
Shcheglovitov A, Vitko I, Bidaud I, Baumgart JP, Navarro-Gonzalez MF, Grayson TH, Lory P, Hill CE, Perez-Reyes E (2008) Alternative splicing within the I–II loop controls surface expression of T-type Cav3.1 calcium channels. FEBS Lett 582(27):3765–3770. doi:10.1016/j.febslet.2008.10.013
Taylor JT, Huang L, Pottle JE, Liu K, Yang Y, Zeng X, Keyser BM, Agrawal KC, Hansen JB, Li M (2008) Selective blockade of T-type Ca2+ channels suppresses human breast cancer cell proliferation. Cancer Lett 267(1):116–124. doi:10.1016/j.canlet.2008.03.032
Tzeng BH, Chen YH, Huang CH, Lin SS, Lee KR, Chen CC (2012) The Ca(v)3.1 T-type calcium channel is required for neointimal formation in response to vascular injury in mice. Cardiovasc Res 96(3):533–542. doi:10.1093/cvr/cvs257
Uebele VN, Gotter AL, Nuss CE, Kraus RL, Doran SM, Garson SL, Reiss DR, Li Y, Barrow JC, Reger TS, Yang ZQ, Ballard JE, Tang C, Metzger JM, Wang SP, Koblan KS, Renger JJ (2009) Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest 119(6):1659–1667. doi:10.1172/JCI36954
Uhrenholt TR, Schjerning J, Vanhoutte PM, Jensen BL, Skott O (2007) Intercellular calcium signaling and nitric oxide feedback during constriction of rabbit renal afferent arterioles. Am J Physiol Renal Physiol 292(4):F1124–F1131. doi:10.1152/ajprenal.00420.2006
Vitko I, Bidaud I, Arias JM, Mezghrani A, Lory P, Perez-Reyes E (2007) The I–II loop controls plasma membrane expression and gating of Cav3.2 T-type Ca2+ channels: a paradigm for childhood absence epilepsy mutations. J Neurosci 27(2):322–330
Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, Shin HS, Wu S, Isakson BE, Witzenrath M, de Wit C, Fleming I, Kuppe H, Kuebler WM (2012) Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest 122(11):4218–4230. doi:10.1172/JCI59176
Wu S, Haynes J Jr, Taylor JT, Obiako BO, Stubbs JR, Li M, Stevens T (2003) Cav3.1 (α1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circ Res 93(4):346–353
Acknowledgments
We would like to thank Lis Teusch and Vivi Monrad for expert technical assistance. Also, we thank Philippe Lory, University de Montpellier, INSERM U661, France for back crossing the Cav3.1−/− mice into a C57BL/6 background. This work was supported by grants from the Danish Medical Research Council (11-107552), The Danish Heart Foundation (11-04-R84-A3492-22663) and Aase and Ejnar Danielsens Foundation. The bioimaging experiments reported in this paper were performed at DaMBIC, a bioimaging research core facility, at the University of Southern Denmark. DaMBIC was established by an equipment grant from the Danish Agency for Science Technology and Innovation and by internal funding from the University of Southern Denmark
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svenningsen, P., Andersen, K., Thuesen, A.D. et al. T-type Ca2+ channels facilitate NO-formation, vasodilatation and NO-mediated modulation of blood pressure. Pflugers Arch - Eur J Physiol 466, 2205–2214 (2014). https://doi.org/10.1007/s00424-014-1492-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-014-1492-4