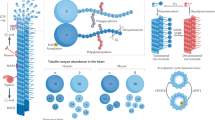
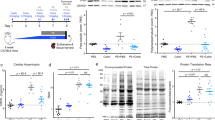
Abstract
Microtubules are a major component of the cardiac myocyte cytoskeleton. Interventions that alter it may influence cardiac mechanical and electrical activity by disrupting the trafficking of proteins to and from the surface membrane by molecular motors such as dynein, which use microtubules as tracks to step along. Free tubulin dimers may transfer GTP to the α-subunits of G-proteins, thus an increase in free tubulin could increase the activity of G-proteins; evidence for and against such a role exists. There is more general agreement that microtubules act as compression-resisting structures within myocytes, influencing visco-elasticity of myocytes and increasing resistance to shortening when proliferated and resisting deformation from longitudinal shear stress. In response to pressure overload, there can be post-translational modifications resulting in more stable microtubules and an increase in microtubule density. This is accompanied by contractile dysfunction of myocytes which can be reversed by microtubule disruption. There are reports of mechanically induced changes in electrical activity that are dependent upon microtubules, but at present, a consensus is lacking on whether disruption or proliferation would be beneficial in the prevention of arrhythmias. Microtubules certainly play a role in the response of cardiac myocytes to mechanical stimulation, the exact nature and significance of this role is still to be fully determined.



Similar content being viewed by others
References
Allen DG, Blinks JR, Godt RE (1984) Influence of deuterium oxide on calcium transients and myofibrillar responses of frog skeletal muscle. J Physiol 354:225–251
Bailey BA, Dipla K, Li S, Houser SR (1997) Cellular basis of contractile derangements of hypertrophied feline ventricular myocytes. J Mol Cell Cardiol 29:1823–1835
Belmadani S, Pous C, Fischmeister R, Mery PF (2004) Post-translational modifications of tubulin and microtubule stability in adult rat ventricular myocytes and immortalized HL-1 cardiomyocytes. Mol Cell Biochem 258:35–48
Belmadani S, Pous C, Ventura-Clapier R, Fischmeister R, Mery PF (2002) Post-translational modifications of cardiac tubulin during chronic heart failure in the rat. Mol Cell Biochem 237:39–46
Calaghan SC, Le Guennec JY, White E (2001) Modulation of Ca(2+) signaling by microtubule disruption in rat ventricular myocytes and its dependence on the ruptured patch–clamp configuration. Circ Res 88:E32–E37
Calaghan SC, Le Guennec JY, White E (2004) Cytoskeletal modulation of electrical and mechanical activity in cardiac myocytes. Prog Biophys Mol Biol 84:29–59
Calligaris D, Verdier-Pinard P, Devred F, Villard C, Braguer D, Lafitte D (2010) Microtubule targeting agents: from biophysics to proteomics. Cell Mol Life Sci 67:1089–1104
Casini S, Tan HL, Demirayak I, Remme CA, Amin AS, Scicluna BP, Chatyan H, Ruijter JM, Bezzina CR, van Ginneken AC, Veldkamp MW (2010) Tubulin polymerization modifies cardiac sodium channel expression and gating. Cardiovasc Res 85:691–700
Cassimeris L, Tran P (2010) Microtubules in vivo. Methods Cell Biol 97:1–530
Choi WS, Khurana A, Mathur R, Viswanathan V, Steele DF, Fedida D (2005) Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res 97:363–371
Collins JF, Pawloski-Dahm C, Davis MG, Ball N, Dorn GW, Walsh RA (1996) The role of the cytoskeleton in left ventricular pressure overload hypertrophy and failure. J Mol Cell Cardiol 28:1435–1443
Cooper G (2000) Cardiocyte cytoskeleton in hypertrophied myocardium. Heart Fail Rev 5:187–201
Cooper G (2006) Cytoskeletal networks and the regulation of cardiac contractility: microtubules, hypertrophy, and cardiac dysfunction. Am J Physiol Heart Circ Physiol 291:H1003–H1014
David-Pfeuty T, Simon C, Pantaloni D (1979) Effect of antimitotic drugs on tubulin GTPase activity and self-assembly. J Biol Chem 254:11696–11702
Desai A, Mitchison TJ (1997) Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13:83–117
Dick DJ, Lab MJ (1998) Mechanical modulation of stretch-induced premature ventricular beats: induction of mechanoelectric adaptation period. Cardiovasc Res 38:181–191
Dutcher SK (2001) The tubulin fraternity: alpha to eta. Curr Opin Cell Biol 13:49–54
Fassett JT, Xu X, Hu X, Zhu G, French J, Chen Y, Bache RJ (2009) Adenosine regulation of microtubule dynamics in cardiac hypertrophy. Am J Physiol Heart Circ Physiol 297:H523–H532
Gennerich A, Vale RD (2009) Walking the walk: how kinesin and dynein coordinate their steps. Curr Opin Cell Biol 21:59–67
Goldstein MA, Entman ML (1979) Microtubules in mammalian heart muscle. J Cell Biol 80:183–195
Gomez AM, Kerfant BG, Vassort G (2000) Microtubule disruption modulates Ca(2+) signaling in rat cardiac myocytes. Circ Res 86:30–36
Goswami C, Kuhn J, Heppenstall PA, Hucho T (2010) Importance of non-selective cation channel TRPV4 interaction with cytoskeleton and their reciprocal regulations in cultured cells. PLoS ONE 5:e11654
Granzier HL, Irving TC (1995) Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J 68:1027–1044
Harris TS, Baicu CF, Conrad CH, Koide M, Buckley JM, Barnes M, Cooper G, Zile MR (2002) Constitutive properties of hypertrophied myocardium: cellular contribution to changes in myocardial stiffness. Am J Physiol Heart Circ Physiol 282:H2173–H2182
Hein S, Kostin S, Heling A, Maeno Y, Schaper J (2000) The role of the cytoskeleton in heart failure. Cardiovasc Res 45:273–278
Heling A, Zimmermann R, Kostin S, Maeno Y, Hein S, Devaux B, Bauer E, Klovekorn WP, Schlepper M, Schaper W, Schaper J (2000) Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ Res 86:846–853
Hill TL, Kirschner MW (1982) Bioenergetics and kinetics of microtubule and actin filament assembly–disassembly. Int Rev Cytol 78:1–125
Hongo K, Brette F, Haroon MM, White E (2000) Mechanisms associated with the negative inotropic effect of deuterium oxide in single rat ventricular myocytes. Exp Physiol 85:133–142
Houser SR, Piacentino V III, Weisser J (2000) Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol 32:1595–1607
Howarth FC, Calaghan SC, Boyett MR, White E (1999) Effect of the microtubule polymerizing agent taxol on contraction, Ca2+ transient and l-type Ca2+ current in rat ventricular myocytes. J Physiol 516:409–419
Ingber DE (2008) Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol 97:163–179
Ingber DE (2002) Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circ Res 91:877–887
Iribe G, Ward CW, Camelliti P, Bollensdorff C, Mason F, Burton RA, Garny A, Morphew MK, Hoenger A, Lederer WJ, Kohl P (2009) Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ Res 104:787–795
Isenberg G, Kazanski V, Kondratev D, Gallitelli MF, Kiseleva I, Kamkin A (2003) Differential effects of stretch and compression on membrane currents and [Na+]c in ventricular myocytes. Prog Biophys Mol Biol 82:43–56
Ives CL, Eskin SG, McIntire LV (1986) Mechanical effects on endothelial cell morphology: in vitro assessment. In Vitro Cell Dev Biol 22:500–507
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Kerfant BG, Vassort G, Gomez AM (2001) Microtubule disruption by colchicine reversibly enhances calcium signaling in intact rat cardiac myocytes. Circ Res 88:E59–E65
Kohl P, Sachs F, Franz MR (2005) Cardiac mechano-electric feedback and arrhythmias, from pipette to patient. Saunders Elsevier, Philadelphia
Kurachi M, Hoshi M, Tashiro H (1995) Buckling of a single microtubule by optical trapping forces: direct measurement of microtubule rigidity. Cell Motil Cytoskeleton 30:221–228
Lampidis TJ, Kolonias D, Savaraj N, Rubin RW (1992) Cardiostimulatory and antiarrhythmic activity of tubulin-binding agents. Proc Natl Acad Sci USA 89:1256–1260
Larsen TH, Dalen H, Boyle R, Souza MM, Lieberman M (2000) Cytoskeletal involvement during hypo-osmotic swelling and volume regulation in cultured chick cardiac myocytes. Histochem Cell Biol 113:479–488
Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH (2002) Microtubule structure at 8 A resolution. Structure 10:1317–1328
Limas CJ, Limas C (1983) Involvement of microtubules in the isoproterenol-induced ‘down’-regulation of myocardial beta-adrenergic receptors. Biochim Biophys Acta 735:181–184
Loewen ME, Wang Z, Eldstrom J, Dehghani ZA, Khurana A, Steele DF, Fedida D (2009) Shared requirement for dynein function and intact microtubule cytoskeleton for normal surface expression of cardiac potassium channels. Am J Physiol Heart Circ Physiol 296:H71–H83
Lowe J, Li H, Downing KH, Nogales E (2001) Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol 313:1045–1057
Madias C, Maron BJ, Supron S, Estes NA III, Link MS (2008) Cell membrane stretch and chest blow-induced ventricular fibrillation: commotio cordis. J Cardiovasc Electrophysiol 19:1304–1309
Marsh JD, Lachance D, Kim D (1985) Mechanisms of beta-adrenergic receptor regulation in cultured chick heart cells. Role of cytoskeleton function and protein synthesis. Circ Res 57:171–181
Motlagh D, Alden KJ, Russell B, Garcia J (2002) Sodium current modulation by a tubulin/GTP coupled process in rat neonatal cardiac myocytes. J Physiol 540:93–103
Nicolas CS, Park KH, El HA, Camonis J, Kass RS, Escande D, Merot J, Loussouarn G, Le BF, Baro I (2008) IKs response to protein kinase A-dependent KCNQ1 phosphorylation requires direct interaction with microtubules. Cardiovasc Res 79:427–435
Nishimura S, Nagai S, Katoh M, Yamashita H, Saeki Y, Okada J, Hisada T, Nagai R, Sugiura S (2006) Microtubules modulate the stiffness of cardiomyocytes against shear stress. Circ Res 98:81–87
O'Connell A, Calaghan S, White E (2005) Abolition of Gi signalling does not reveal a positive inotropic effect of microtubule disruption in rat ventricular myocytes. J Physiol 569P, PC13
Olmsted JB (1986) Microtubule-associated proteins. Annu Rev Cell Biol 2:421–457
Palmer BM, Valent S, Holder EL, Weinberger HD, Bies RD (1998) Microtubules modulate cardiomyocyte beta-adrenergic response in cardiac hypertrophy. Am J Physiol 275:H1707–H1716
Parker KK, Taylor LK, Atkinson JB, Hansen DE, Wikswo JP (2001) The effects of tubulin-binding agents on stretch-induced ventricular arrhythmias. Eur J Pharmacol 417:131–140
Pascarel C, Brette F, Le Guennec JY (2001) Enhancement of the T-type calcium current by hyposmotic shock in isolated guinea-pig ventricular myocytes. J Mol Cell Cardiol 33:1363–1369
Pitcher JA, Hall RA, Daaka Y, Zhang J, Ferguson SS, Hester S, Miller S, Caron MG, Lefkowitz RJ, Barak LS (1998) The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J Biol Chem 273:12316–12324
Quaile MP, Rossman EI, Berretta RM, Bratinov G, Kubo H, Houser SR, Margulies KB (2007) Reduced sarcoplasmic reticulum Ca(2+) load mediates impaired contractile reserve in right ventricular pressure overload. J Mol Cell Cardiol 43:552–563
Rappaport L, Samuel JL (1988) Microtubules in cardiac myocytes. Int Rev Cytol 113:101–143
Rasenick MM, Stein PJ, Bitensky MW (1981) The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature 294:560–562
Rowinsky EK, Donehower RC (1995) Paclitaxel (taxol). N Engl J Med 332:1004–1014
Sadoshima J, Takahashi T, Jahn L, Izumo S (1992) Roles of mechano-sensitive ion channels, cytoskeleton, and contractile activity in stretch-induced immediate-early gene expression and hypertrophy of cardiac myocytes. Proc Natl Acad Sci USA 89:9905–9909
Sato H, Nagai T, Kuppuswamy D, Narishige T, Koide M, Menick DR, Cooper G (1997) Microtubule stabilization in pressure overload cardiac hypertrophy. J Cell Biol 139:963–973
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667
Schroder EA, Tobita K, Tinney JP, Foldes JK, Keller BB (2002) Microtubule involvement in the adaptation to altered mechanical load in developing chick myocardium. Circ Res 91:353–359
Shiels H, O'Connell A, Qureshi MA, Howarth FC, White E, Calaghan S (2007) Stable microtubules contribute to cardiac dysfunction in the streptozotocin-induced model of type 1 diabetes in the rat. Mol Cell Biochem 294:173–180
Shimoni Y, Rattner JB (2001) Type 1 diabetes leads to cytoskeleton changes that are reflected in insulin action on rat cardiac K(+) currents. Am J Physiol Endocrinol Metab 281:E575–E585
Stamenovic D, Mijailovich SM, Tolic-Norrelykke IM, Chen J, Wang N (2002) Cell prestress. II. Contribution of microtubules. Am J Physiol Cell Physiol 282:C617–C624
Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G (1997) Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res 80:281–289
Takahashi M, Tsutsui H, Tagawa H, Igarashi-Saito K, Imanaka-Yoshida K, Takeshita A (1998) Microtubules are involved in early hypertrophic responses of myocardium during pressure overload. Am J Physiol 275:H341–H348
Tsutsui H, Tagawa H, Kent RL, McCollam PL, Ishihara K, Nagatsu M, Cooper G (1994) Role of microtubules in contractile dysfunction of hypertrophied cardiocytes. Circulation 90:533–555
Wang N, Yan K, Rasenick MM (1990) Tubulin binds specifically to the signal-transducing proteins, Gs alpha and Gi alpha 1. J Biol Chem 265:1239–1242
Watson PA, Hannan R, Carl LL, Giger KE (1996) Contractile activity and passive stretch regulate tubulin mRNA and protein content in cardiac myocytes. Am J Physiol 271:C684–C689
Webster DR, Patrick DL (2000) Beating rate of isolated neonatal cardiomyocytes is regulated by the stable microtubule subset. Am J Physiol Heart Circ Physiol 278:H1653–H1661
Xiao J, Cao H, Liang D, Liu Y, Zhang H, Zhao H, Liu Y, Li J, Yan B, Peng L, Zhou Z, Chen YH (2010) Taxol, a microtubule stabilizer, prevents ischemic ventricular arrhythmias in rats. J Cell Mol Med. doi:10.1111/j.1582-4934.2010.01106.x
Xiao J, Zhang H, Liang D, Liu Y, Liu Y, Zhao H, Li J, Peng L, Chen YH (2010) Taxol, a microtubule stabilizer, prevents atrial fibrillation in in vitro atrial fibrillation models using rabbit hearts. Med Sci Monit 16:BR353–BR360
Yamamoto S, Tsutsui H, Takahashi M, Ishibashi Y, Tagawa H, Imanaka-Yoshida K, Saeki Y, Takeshita A (1998) Role of microtubules in the viscoelastic properties of isolated cardiac muscle. J Mol Cell Cardiol 30:1841–1853
Yin FC (1981) Ventricular wall stress. Circ Res 49:829–842
Yutao X, Geru W, Xiaojun B, Tao G, Aiqun M (2006) Mechanical stretch-induced hypertrophy of neonatal rat ventricular myocytes is mediated by beta(1)-integrin-microtubule signaling pathways. Eur J Heart Fail 8:16–22
Zile MR, Green GR, Schuyler GT, Aurigemma GP, Miller DC, Cooper G (2001) Cardiocyte cytoskeleton in patients with left ventricular pressure overload hypertrophy. J Am Coll Cardiol 37:1080–1084
Zile MR, Koide M, Sato H, Ishiguro Y, Conrad CH, Buckley JM, Morgan JP, Cooper G (1999) Role of microtubules in the contractile dysfunction of hypertrophied myocardium. J Am Coll Cardiol 33:250–260
Zile MR, Richardson K, Cowles MK, Buckley JM, Koide M, Cowles BA, Gharpuray V, Cooper G (1998) Constitutive properties of adult mammalian cardiac muscle cells. Circulation 98:567–579
Acknowledgements
The author thanks Dr. Sarah Calaghan for comments on this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
White, E. Mechanical modulation of cardiac microtubules. Pflugers Arch - Eur J Physiol 462, 177–184 (2011). https://doi.org/10.1007/s00424-011-0963-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-011-0963-0