Abstract
We previously reported that hydrogen sulfide (H2S) preconditioning (SP) produces cardioprotective effects against ischemia in rat cardiac myocytes. The present study aims to elucidate the signaling mechanisms involved in SP-induced cardioprotection by investigating the role of extracellular signal regulated kinase (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/Akt. We found that preconditioning with NaHS (a H2S donor) for three cycles significantly decreased myocardial infarct size and improved heart contractile function in the isolated rat hearts. NaHS (1–100 μM) concentration-dependently increased cell viability and percentage of rod-shaped cardiac myocytes. Blockade of ERK1/2 with PD 98059 or PI3K/Akt with LY-294002 or Akt inhibitor III during either preconditioning or ischemia periods significantly attenuated the cardioprotection of SP, suggesting that both ERK1/2 and PI3K/Akt triggered and mediated the cardioprotection of SP. Moreover, SP induced ERK1/2 and Akt phosphorylation in isolated hearts. The phosphorylation of ERK1/2 induced by SP was attenuated by either glibenclamide, an ATP-sensitive K+ channel (KATP) blocker, or chelerythrine, a specific protein kinase C (PKC) blocker. In addition, ischemic-preconditioning-induced ERK1/2 activation was reversed by inhibiting endogenous H2S production, suggesting that ERK1/2 activation induced by ischemic preconditioning was, at least partly, mediated by endogenous H2S. In conclusion, KATP/PKC/ERK1/2 and PI3K/Akt pathways contributed to SP-induced cardioprotection.
Similar content being viewed by others
Introduction
Ever since its discovery in 1986 by Murry et al. [22], ischemic preconditioning (IP) has been positioned as one of the most potent cardioprotective mechanisms in line with ischemic postconditioning, which was later introduced in 2003 by Zhao et al. [35]. In brief, IP refers to the phenomenon whereby brief periods of sublethal ischemic episodes protect the tissue from subsequent more severe ischemia-reperfusion insults [7]. In the heart, this phenomenon is of therapeutic importance considering the high mortality and morbidity of ischemic heart diseases. The protective effect of IP in the heart is manifested in vivo and ex vivo by a reduction in infarct size, restoration of cardiac pump function, and alleviation of ventricular arrhythmia, and in vitro by improving cardiac myocyte contractility and reducing rates of cell necrosis and apoptosis [7]. Despite 20 years of research into the field of ischemic preconditioning, the actual mechanism of protection remains unclear [15]. Various signal-transduction pathways have been identified as mediators of IP. PKC and KATP channels have gained much attention to serve as the central mediator of IP, although KATP channel was also shown to play certain role as a trigger as well as an end-effector (reviewed by [25, 31]). Recently, much progress has been made in elucidating the signal transduction pathways that convey the extracellular signal initiated by the preconditioning stimuli to the intracellular targets of cardioprotection, with many of these pathways involving the activation of a diverse array of survival protein kinase cascades. Among the known cell survival pathways, the mitogen-activated protein kinase (MAPK)/ERK1/2 and PI-3-kinase (phosphatidylinositol 3-kinase)/Akt pathways are of considerable importance [15].
H2S, originally known as a noxious gas toxic to the central nervous and respiratory systems, has recently been found to be generated in situ in various tissues and to exert physiological functions [33, 34]. Thus, H2S is now viewed as a third candidate of the so-called “gaseous transmitters”, alongside nitric oxide (NO) and carbon monoxide (CO). In the heart, H2S is produced in the myocardium, fibroblasts, and blood vessels from l-cysteine by the enzyme cystathionine γ-lyase (CSE) and accumulates at relatively high local concentrations [10]. Convincing evidence has shown that H2S produces a negative inotropic effect in the heart [10] and regulates vascular tone by opening KATP channels [34] and acting in synergy with NO [17]. These various actions highlight the potential importance of endogenous H2S for the physiological regulations of cardiovascular system function.
Our recent work [3, 26] has shown that H2S preconditioning (SP) produced cardiac protective effects similar to that induced by IP, and interestingly, endogenous H2S contributes to metabolic inhibition preconditioning-induced cardioprotection. The underlying mechanism(s) may involve NO release, PKC activation, and KATP channel opening [26], but still remains to be clarified. It has been reported previously that MAPK activation is a trigger for protection induced by IP [21]. Although discrepancies exist in the role of other MAPKs members [(p38-MAPK and c-Jun NH2-terminal kinases1/2 (JNK1/2)] in IP in different animal models [15], accumulating data suggest that the MAP kinase kinase (MEK1/2) extracellular signal-regulated kinase (ERK1/2 or p44/p42-MAPK) cascade is a pro-survival mechanism and is downstream of PKCɛ in the setting of IP [1, 8]. A recent study has also demonstrated that ERK1/2 is activated by the opening of the mitoKATP channels [23]. These findings position ERK1/2 as a converging point for PKC and KATP channels, both of which have been identified in our previous studies as indispensable in H2S preconditioning [3, 26]. In addition, IP may also protect the heart via another survival kinase mechanism, the phosphatidylinositol 3-kinase (PI3K)-Akt (also called PKB) cascade [15]. Thus, in this study, we determined the phosphorylation of ERK1/2 and Akt upon SP and investigated their roles in the cardioprotection induced by SP.
Materials and methods
The study protocol was approved by the Institutional Animal Care and Use Committees (IACUC) of National University of Singapore.
Preparation of Langendorff-perfused rat hearts
Adult male Sprague–Dawley rats (8 weeks, 190–250 g) were anesthetized by intraperitoneal (i.p.) administration of pentobarbital (60 mg/kg). Heparin (1,000 IU) was administered i.p. to prevent coagulation during removal of the heart. A central thoracotomy was performed. The heart was removed and perfused in a Langendorff system with Krebs–Henseleit (K–H) buffer (containing NaCl 118 mM, KCl 5 mM, MgSO4 1.2 mM, KH2PO4 1.2 mM, CaCl2 1.25 mM, NaHCO3 25 mM, and glucose 11 mM, pH 7.4, constant flow rate 12 ml/min) at 37°C for 10 min.
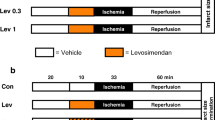
Electrocardiogram (ECG) of the isolated heart was monitored and recorded with two electrodes hooked to the apex and the aorta, respectively, as described previously [3]. Isolated hearts were allowed to stabilize for 10 min. Any heart that exhibited arrhythmias during this period was discarded. Isolated rat hearts with normal ECGs were randomly assigned to the following experiment groups. The experimental protocol is shown in Fig. 1. In vehicle preconditioning (VP) group, hearts were perfused with K–H buffer for 34 min and subjected to 35-min global ischemia (perfusion rate = 0 ml/min) for Western blotting analysis or regional ischemia (ligation of the left main coronary artery, LAD) for myocardial infarction assessment. The hearts were then reperfused with K–H solution for 1 h. In SP group, the hearts were subjected to three cycles of SP, each consisting of 3-min NaHS (100 μM) perfusion separated by 5-min K–H buffer perfusion, and then subjected to 35-min ischemia and reperfusion. To determine the involvement of PKC or KATP channels in the activation of ERK1/2, chelerythrine (Che, 1 μM) or glibenclamide (Gliben, 10 μM) was applied to the perfusion solution 10 min before and during SP, respectively (Fig. 1 Che/Gliben). In the IP group, the hearts received three cycles of IP, each consisting of 3-min non-flow global ischemia separated by 5-min reperfusion before being subjected to 35-min global ischemia followed by 1-h reperfusion (Fig. 1, IP). To determine the involvement of endogenous H2S, hearts were exposed to PAG (2 mM) or BCA (10 mM) 10 min before and during IP (Fig. 1, PAG/BCA).
Experimental protocols for the cardioprotection of SP and IP in isolated rat hearts and cardiac myocytes. Open fields K–H solution at perfusion rate of 13 ml/min in isolated hearts or DMEM solution in isolated cardiac myocytes; solid fields ligation of LAD (for myocardial infarction assessment in isolated hearts), full-stop perfusion of K–H buffer (for contractile function assessment in isolated hearts), or simulated ischemia buffer (for cell viability and morphology in cardiac myocytes); slashed fields K–H solution (hearts) with NaHS or DMEM solution (cardiac myocytes) with NaHS
Measurement of myocardial infarction size
After reperfusion for 1 h, the infarct–risk volume ratio was determined. As described previously [13], the heart was stained by slowly infusing 1 ml Evan’s blue (3% w/v in phosphate-buffered saline) via the aorta, followed by perfusion with K–H solution to wash out unbound stains. The heart was then immediately removed from the perfusion apparatus, weighed, and stored overnight at −20°C. The frozen heart was thereafter cut into six or seven transverse sections (approximately 1 mm in thickness) across the long axis, stained with 1% TTC in phosphate buffer (pH 7.4) for 15 min at 37°C, and then fixed in 10% formalin overnight before slides of heart tissue were measured using a computer morphometry system (Bioquant 98). The size of the myocardial infarction (appearing as pale color) was quantified and calculated as percentage of area at risk which is the area not stained by Evan’s blue.
Measurement of contractile function
To measure left ventricular pressure, a latex balloon was inserted into the left ventricle (LV) via a left atrial incision. The balloon was filled with bubble-free saline and attached to a pressure transducer connected to a Powerlab system (Australia). The balloon volume was set to produce a left ventricular end-diastolic pressure (LVEDP) of 5 to 10 mmHg during the initial equilibration. The isovolumic measurement of left ventricular developed pressure (LVDP, which was calculated from the difference between left ventricular peak systolic pressure and LVEDP) was employed as the index of LV contraction.
Preparation of isolated rat cardiac myocytes
Cardiac myocytes were isolated from the heart by a collagenase perfusion method as described previously [3]. The heart was removed and perfused in a Langendorff system with K–H buffer (containing NaCl 118 mM, KCl 5 mM, MgSO4 1.2 mM, KH2PO4 1.2 mM, CaCl2 1.25 mM, NaHCO3 25 mM, and glucose 11 mM, pH 7.4, constant flow rate 12 ml/min) at 37°C for 10 min. Collagenase (type II) and protease were then added to the calcium-free Tyrode’s buffer to a concentration of 0.84 and 0.28 mg/ml, respectively. After 25–30 min of perfusion with collagenase and protease, the heart was perfused with Tyrode’s solution containing 0.25 mM CaCl2 for 5 min. The ventricular tissue was then transferred to a dish filled with oxygenated Tyrode’s solution (with 0.25 mM CaCl2) and cut into small pieces with scissors. Then, the solution was gently stirred with a glass rod for 5 min to separate the cardiac myocytes from each other. The residue was filtered through a 250-μm mesh screen to remove undigested tissues. Cardiac myocytes were then sedimented by centrifugation at 100 × g for 1 min. More than 70% of the cells were rod shaped and impermeable to trypan blue. The Ca2+ concentration of the Tyrode’s solution was then increased gradually to 1.25 mM in 45 min. The myocytes were then allowed to stabilize at room temperature for 30 min.
The consequences of ischemia include metabolic inhibition, hypoxia, and acidosis. For in vitro studies, an “ischemia buffer” was used to simulate the internal environment during local ischemia. The severe “ischemia buffer” [11] consisted of 137 mM NaCl, 15.8 mM KCl, 0.49 mM MgCl2, 0.9 mM CaCl2, 4 mM HEPES, supplemented with 20 mM 2-deoxyglucose (2-DOG, an inhibitor of glycolysis), 2.5 mM sodium dithionite (Na2S2O4, an oxygen scavenger), and 20 mM sodium lactate (to mimic local lactate accumulation), pH 6.5 (to mimic acidosis). The experimental protocol was shown in Fig. 1. In VP group, cells were incubated in DMEM medium for 34 min and then subjected to simulated ischemia for 35 min followed by 10-min reperfusion. For the preconditioning group, cells were subjected to three cycles of 3 min NaHS (100 μM, SP) separated by 5 min fresh DMEM culture medium. After SP, cells were subjected to severe “ischemia buffer” for 35 min and 10-min reperfusion with DMEM culture medium. To determine whether ERK1/2 or PI3K/Akt serves as a trigger, PD 98059 (10 μM) or LY 294002 (15 μM) was added to the perfusion fluid 10 min before and during SP, respectively (Fig. 1; ERK-t/Akt-t). To determine whether ERK1/2 or PI3K/Akt serves as a mediator, PD 98059 (10 μM) or LY 294002 (15 μM) was given 5 min before and during ischemia, respectively, (Fig. 1; ERK-m/Akt-m).
Measurement of cell viability and morphology
After 10-min reperfusion, cell viability and morphology were observed. Trypan blue exclusion was used as an index of myocyte viability [36]. After the live cells were incubated with 0.4% (w/v) trypan blue dye for 3 min, those that were unstained were termed to be non-blue cells. Non-blue cells/total cells were determined in a hemocytometer chamber using a light microscope (10× magnification).
Cell morphology was assessed by microscopic examination [36]. The percentage of rod-shaped cells was counted as an index of cytoskeleton integrity and cell injury. Rod-shape was defined as the ratio of length–width to be >3.
Detection of ERK1/2 and Akt phosphorylation
Rat hearts were removed and perfused in a Langendorff system with K–H buffer (containing NaCl 118 mM, KCl 5 mM, MgSO4 1.2 mM, KH2PO4 1.2 mM, CaCl2 1.25 mM, NaHCO3 25 mM, and glucose 11 mM, pH 7.4, constant flow rate 12 ml/min) at 37°C for 10 min. In VP group, hearts were perfused with K–H buffer for 34 min and subjected to 35-min global ischemia (perfusion rate = 0 ml/min). The hearts were then reperfused with K–H solution for 1 h. In SP and IP groups, the hearts were subjected to three cycles of SP or IP, each consisting of 3-min NaHS (100 μM) perfusion (SP) or global ischemia (IP), separated by 5-min K–H buffer perfusion, and then subjected to 35-min ischemia and reperfusion. After 10-min reperfusion, hearts were snap-frozen in liquid nitrogen and homogenized in ice-cold lysis buffer [Tris pH 7.4, 10 mM; NaCl 100 mM; EDTA 1 mM; EGTA 1 mM; NaF 1 mM; Na4P2O7 20 mM; Na3VO4 2 mM; sodium dodecyl sulfate (SDS) 0.1%; deoxycholic acid 0.5%; Triton-X100 1%; glycerol 10%] supplemented with protease inhibitor cocktail tablet (Roche). After centrifugation at 10,000 × g for 10 min, the supernatant was collected and denatured with SDS-sample buffer, and epitopes were exposed by boiling the protein samples at 100°C water for 10 min. Equal amount of proteins were loaded and separated by electrophoresis on 10% SDS-polyacrylamide gel. Then, the protein bands were transferred to a polyvinylidene difluoride membrane. The membrane was probed first with the primary antibody that recognizes phospho-p42/p44 MAPK and total-p42/p44 MAPK antibodies and then with a horseradish peroxidase-conjugated goat-anti-rabbit IgG secondary antibody. Immunoreactivity was detected using the enhanced chemiluminescence method.
Reagents and chemicals
NaHS, chelerythrine chloride, dl-propargylglycine (PAG), β-cyano-l-alanine (BCA), triphenyl tetrazolium chloride (TTC), Evans’ blue and in vitro LDH assay kit were purchased from Sigma Chemical, USA. Glibenclamide was obtained from Tocris Cookson, UK. PD 98059, LY 294002 and Akt inhibitor III (Akt-i) were obtained from EMD Biosciences, Germany. Monoclonal anti-phospho-p42/p44 MAPK rabbit IgG and monoclonal anti-total-p42/p44 MAPK rabbit IgG, phospho-Akt (Ser473) and total Akt antibodies were purchased from Cell Signaling Technology, USA. Glibenclamide, PD 98059, LY 294002, and Akt-i were dissolved in dimethyl sulphoxide (DMSO) and added to the Krebs–Henseleit buffer or DMEM medium such that the final DMSO concentration was less than 0.1%. All other chemicals were dissolved in distilled water.
As used in numerous publications, NaHS, a donor of H2S, was employed in these experiments, as its use allows for a better definition of the concentration of H2S in solution than bubbling H2S gas. NaHS dissociates to Na+ and HS− in solution. Thereafter, HS− associates with H+ and produces H2S. Approximately one third of the H2S in aqueous solution exists in the undissociated form (H2S) at 20°C [33]. At 37°C, the undissociated form of H2S is around 18.5%, i.e., approximately 18.5 μM from 100 μM NaHS [6], which is well within the range of physiological concentrations in the plasma [33].
Statistical analysis
Values presented are mean±standard error of mean. One-way analysis of variance followed by post hoc Turkey test was used to determine the difference between groups. The significance level was set at P < 0.05.
Results
Cardioprotective effects of SP in the rat cardiac myocytes and isolated hearts
We first examined the concentration-dependent effect of the cardioprotective effect of H2S preconditioning. The experimental procedure is shown in Fig. 1 and described in “Materials and methods”. As shown in Fig. 2a and b, preconditioning with NaHS at a concentration range of 1–100 μM concentration-dependently increased cell viability and percentage of rod-shaped cardiac myocytes. The maximum response was observed when NaHS was at 100 μM. Therefore, 100 μM was chosen for all other experiments.
Cardioprotective effects of SP on ischemia-induced injury. a–b Concentration-dependent effects of NaHS on cell viability (a) and percentage of rod-shaped cells (b) in cardiac myocytes. Values were mean±SEM, n = 6–8 preparations from different rat hearts. *P < 0.05, **P < 0.01, ***P < 0.001 vs the respective value in VP group (NaHS = 0 M); c–e Cardioprotection of SP on myocardial infarct size (c), LVDP (d), and LVEDP (e) in isolated rat hearts. Values were mean±SEM. n = 7–10. *P < 0.05, ***P < 0.001 vs the respective value in VP group
To confirm whether SP can also protect heart tissue damage, we observed the cardioprotection of SP on myocardial infarction and heart contractile function. As shown in Fig. 2c, after 1-h reperfusion, SP significantly decreased infarct size as compared with VP. The effect of SP on heart contractile function was also observed. As shown in Fig. 2d and e, SP increased LVDP and decreased LVEDP after reperfusion for 20 min. These data confirm that preconditioning of the heart with NaHS at 100 μM significantly protected heart against ischemic injury.
Role of ERK1/2 in the cardioprotection of SP
We further determined whether ERK1/2 mediates the cardioprotection afforded by SP. As shown in Fig. 3, SP significantly increased cell viability (Fig. 3a) and the percentage of rod-shaped cardiac myocytes (Fig. 3b). To determine whether ERK1/2 serves as a trigger or a mediator in the cardioprotection of SP, PD 98059, a selective ERK1/2 inhibitor, was given 10 min before and during three cycles of SP ERK-t or 5 min before and during ischemia ERK-m respectively. As shown in Fig. 3, PD 98059 with both treatment protocols significantly reversed the cardioprotective effects of SP, suggesting that SP-induced ERK1/2 activation triggered and mediated the cardioprotection of SP.
Cardioprotective effects of SP on cell viability (a) and morphology (b) in the presence and absence of an ERK1/2 inhibitor in the isolated cardiac myocytes. PD-t: PD 98059 was administered 10 min before and during three cycles of SP. PD-m: PD 98059 was administered 5 min before and during ischemia. Values were mean±SEM, n = 5–16 preparations from different rat hearts. ***P < 0.001 vs respective value in VP group; # P < 0.05, ### P < 0.001 vs respective value in SP group
Effect of SP on ERK1/2 kinase activity in the presence and absence of a PKC or a KATP channel blocker in the isolated rat hearts
To further confirm the activation of ERK1/2 by SP, the phosphorylation of ERK1/2 upon SP was determined by Western blotting. As shown in Fig. 4, SP caused significant ERK1/2 phosphorylation but had no effect on total ERK1/2 at the end of 10 min reperfusion. To determine the signaling mechanism for the SP-induced activation of ERK kinases, chelerythrine, a selective PKC inhibitor, or glibenclamide, a KATP channel inhibitor, was used. As shown in Fig. 4, both drugs significantly attenuated the SP-induced ERK1/2 activation, suggesting that the phosphorylation of ERK1/2 is secondary to the activation of PKC and KATP channels.
Effect of SP on phosphorylation of ERK1/2 in the presence and absence of a PKC inhibitor or a KATP channel blocker in the isolated rat hearts. a Representing Western blots of phospho-ERK1/2 and total ERK1/2. b and c Group results showing that both chelerythrine (Che) and glibenclamide (Gliben) significantly attenuated SP-induced phosphorylation of ERK1/2. Values were mean±SEM, n = 4. *P < 0.05 vs respective value in VP group; # P < 0.05, ## P < 0.01 vs respective values in SP group
Effect of IP on ERK1/2 activation in the presence and absence of CSE inhibitors in the isolated rat hearts
As shown in Fig. 5, IP also induced ERK1/2 phosphorylation, but had no effect on total ERK1/2 at the end of 10-min reperfusion. To determine whether the activation of ERK1/2 was mediated by endogenous H2S, PAG, and BCA, two CSE inhibitors were used. PAG or BCA was perfused 15 min before and during IP. As shown in Fig. 5, both PAG and BCA significantly attenuated the activation of ERK1/2 induced by IP. These data suggest that endogenous H2S contributed to the activation of ERK kinase activation during IP.
Effect of IP on ERK1/2 phosphorylation in the presence and absence of CSE inhibitors in the isolated rat hearts. a Representative Western blots of phospho-ERK1/2 and total ERK1/2. b and c PAG and BCA significantly attenuated IP-induced phosphorylation of ERK1/2 (b p44-ERK; c p42-ERK). Values were mean±SEM, n = 4. **P < 0.01, ***P < 0.001 vs the respective value in VP group; ## P < 0.01, ### P < 0.001 vs the respective value in SP group
Effects of SP on PI3K/Akt signaling
In view of the significant role of PI3K/Akt pathway in the cardioprotection of IP, we also determined whether the PI3K/Akt pathway functions in the cytoprotective effect of SP in myocardial ischemia. To evaluate Akt activity after myocardial ischemia, we assessed Akt activity and phosphorylation of its downstream targets using immunoblotting analysis with phospho-specific antibodies. Phosphorylation of Akt at Ser-473, which is required for Akt activation, was measured. As shown in Fig. 6a and b, SP induced phosphorylation of Akt-Ser-473 without changing total Akt protein levels, suggesting that SP activates Akt signaling pathway.
Akt phosphorylation induced by SP and its role in the cardioprotection of SP. a and b Representative Western blots (a) and mean data of the relative intensity (b) of phosphor-Akt and total-Akt upon SP treatment. Mean±SEM, n = 6. **P < 0.01 vs VP. c and d LY 294002 and Akt-i significantly attenuated the cardioprotective effect of SP on cell viability (c), percentage of rod-shaped cells/total number of cells (d). LY-t/AKT-t: LY 294002 or Akt-i was administered 10 min before and during three cycles of SP. LY-m/Akt-m: LY 294002 or Akt-i was administered 5 min before and during ischemia. Mean±SEM, n = 6–22, ***P < 0.001 vs VP; # P < 0.05, ## P < 0.01, ### P < 0.001 vs the respective value in SP group
We further observed the role of PI3K/Akt pathway in the cardioprotection of SP. The specific PI3K/Akt pathway inhibitor (LY-294002 or Akt-i) was given 10 min before and during three cycles of SP (trigger, Fig. 1; Akt-t) or 5 min before and during ischemia (mediator, Fig. 1; Akt-m) to test whether PI3K/Akt serves as a trigger or a mediator. As shown in Fig. 6c and d, treatment with either LY 294002 (15 μM) or Akt-i (10 μM) with the two experimental protocols significantly attenuated the protective effects of SP on both cell viability (Fig. 6c) and morphology (Fig. 6d). Taken together, our data suggest that PI3K/Akt may also contribute to the cardioprotection afforded by SP as both a trigger and a mediator besides ERK1/2.
Discussion
The most important findings in the present study include: (1) SP protected heart and cardiac myocytes against ischemic injury; (2) cardioprotection induced by SP involved activation of ERK1/2; (3) blockade of either KATP or PKC reversed the SP-induced ERK1/2 phosphorylation, suggesting that opening of KATP channels and activation of PKC are pre-requisite for SP-induced ERK1/2 activation; (4) IP induced ERK1/2 activation was attenuated by inhibition of endogenous H2S production, confirming that endogenous H2S contributes to the IP-induced cardioprotection; (5) PI3k/Akt pathway contributed to the cardioprotection induced by SP.
ERK1/2 belongs to the MAPKs family which includes ERK1/2 and stress-activated protein kinases (SAPKs) containing JNK1/2 and p38-MAPK. It is commonly accepted that phosphorylation of ERK1/2 in the cardiac myocytes during early reperfusion serves as a defense mechanism against ischemic stress stimuli [32]. This defense mechanism can be magnified by IP and several forms of pharmacologically induced preconditioning [13, 29]. In this study, we observed that SP protected the heart against ischemia reperfusion injury by reducing myocardial injury. Blockade of ERK1/2 with PD 98059 during either preconditioning or ischemia periods reversed these cardioprotective effects, suggesting that ERK1/2 at least partly triggered and mediated the cardioprotection afforded by SP. This was further confirmed by our Western blot study where SP significantly increased the fraction of phosphorylated ERK1/2 without changing the expression of total ERK1/2. The H2S-induced activation of ERK1/2 is also consistent with two recent findings reporting that sustained exposure to exogenous H2S activates ERK1/2 in vascular smooth muscle cells [30] and in macrophages [24].
The crosstalk between PKC and MEK1/2-ERK1/2 pathways has been studied previously, but still remains controversial in different preconditioning models. It was found that the activation of ERK1/2 was abrogated by PKC inhibition in an in vivo IP model of rabbits [27]. Later on, Heidkamp et al. [16] found that ERK1/2 was mediated by PKCɛ. However, it was also found that ERK activation induced by desflurane preconditioning is independent of PKC [29]. In this study, we found that blockade of PKC greatly attenuated the SP-induced ERK1/2 activation, indicating that PKC activation is a pre-requisite for ERK1/2 phosphorylation during SP. Activation of ERK1/2 may further facilitate the formation of PKCɛ–ERK modules in the mitochondria and subsequently execute cardioprotection signals via phosphorylation and inactivation of the pro-apoptotic proteins, i.e., BAD [2].
Interestingly, we found in the present study that KATP channel is also activated upstream of ERK1/2 during SP. Recently, much research has been focused on the central role of KATP as both a trigger and a distal effector in IP [12, 18, 20]. The most direct known mechanism of KATP-conveyed protection is by shortening action potential duration and by preventing calcium overload [12]. However, KATP also exerts cardioprotective effect by exhibiting more complicated cross talks with other rescuing pathways. Previous studies have shown that opening of KATP channels and activation of PKC may be codependent during IP [9, 19]. The crosstalk among KATP, PKC, and ERK1/2 observed in the present study suggests that like other preconditioning events, SP may orchestrate the vast network of rescuing pathways to execute the prosurvival signals.
Another interesting finding in the present study is that endogenous H2S production contributed to the activation of ERK1/2 induced by IP. This is consistent with our previous findings that endogenous H2S may also protect cardiac myocyte function and cell viability from ischemic injury [3, 26]. However, Sivarajah et al. [28] recently reported that endogenous H2S may not be involved in the cardioprotection of IP on myocardial infarction size in rat in vivo. If this is the case, these data imply that endogenous H2S contribute to the cardioprotection of IP on cellular/functional injury, but may play little role in alleviating severe tissue structural damage during myocardial infarction.
Activation of the PI3K/Akt pathway has been demonstrated to play a key role in both early and delayed myocardial preconditioning [13–15]. PI3K converts phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-trisphosphate, which stimulates phosphorylation of the serine-threonine kinase Akt by phosphoinositide-dependent kinase 1. Akt inhibits formation of the proapoptotic proteins Bad, Bax, and caspase [4] and increases the formation of nitric oxide [5]. Therefore, the PI3K signaling cascade may contribute to the recruitment of multiple endogenous cardioprotective pathways to reduce myocardial damage after ischemia and reperfusion. We found in the present study that SP induced a significant phosphorylation of Akt in the isolated hearts, and, more importantly, the cardioprotective effects of SP were greatly attenuated by inhibition of PI3K and Akt during both preconditioning and ischemia periods. These data suggest that similar as ERK1/2, PI3K/Akt may also serve as a trigger and a mediator in SP-induced cardioprotection.
In conclusion, we found for the first time that both ERK1/2 and PI3K/Akt triggered and mediated the cardioprotection afforded by SP. Activation of both KATP and PKC during SP is necessary to stimulate ERK1/2. Endogenous H2S mediated the cardioprotective effects of IP via activating ERK1/2. Our study provides further evidence for the role of H2S in regulating cardiovascular integrity.
Abbreviations
- 2-DOG:
-
2-deoxy-d-glucose
- Akt-i:
-
Akt inhibitor III
- BCA:
-
β-cyano-l-alanine
- Che:
-
chelerythrine
- CO:
-
carbon monoxide
- CSE:
-
cystathionine γ-lyase
- DMSO:
-
dimethyl sulphoxide
- ERK:
-
extracellular signal regulated kinase
- H2S:
-
hydrogen sulfide
- IP:
-
ischemic preconditioning
- JNK:
-
c-Jun NH2-terminal kinases
- KATP :
-
ATP-sensitive K+ channel
- LAD:
-
left anterior descending coronary artery
- MAPK:
-
mitogen-activated protein kinase
- MEK:
-
MAPK/ERK kinase, or MAP kinase kinase
- Na2S2O4 :
-
sodium dithionite
- NaHS:
-
sodium hydrogen sulfide
- NO:
-
nitric oxide
- PAG:
-
dl-propargylglycine
- PI3K:
-
phospatidylinositol 3-kinase
- PKC:
-
protein kinase C
- SP:
-
NaHS preconditioning
- TTC:
-
triphenyl tetrazolium chloride
- VP:
-
vehicle preconditioning group
References
Armstrong SC (2004) Protein kinase activation and myocardial ischemia/reperfusion injury. Cardiovasc Res 61:427–436
Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, Bolli R, Ping P (2002) Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res 90:390–397
Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK (2006) Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316:670–678
Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–605
Dombkowski RA, Russell MJ, Olson KR (2004) Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol 286:R678–R685
Eisen A, Fisman EZ, Rubenfire M, Freimark D, McKechnie R, Tenenbaum A, Motro M, Adler Y (2004) Ischemic preconditioning: nearly two decades of research. A comprehensive review. Atherosclerosis 172:201–210
Gao Y, Wang Y, Shan YQ, Pan MX (2003) PKC-dependent activation of P44/42 MAPKs and HSP70 in signal transduction pathways during hepatocyte ischemic preconditioning. Zhonghua Yi Xue Za Zhi 83:242–246
Gaudette GR, Krukenkamp IB, Saltman AE, Horimoto H, Levitsky S (2000) Preconditioning with PKC and the ATP-sensitive potassium channels: a codependent relationship. Ann Thorac Surg 70:602–608
Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C (2004) H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313:362–368
Gordon JM, Dusting GJ, Woodman OL, Ritchie RH (2003) Cardioprotective action of CRF peptide urocortin against simulated ischemia in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol 284:H330–H336
Gross GJ, Peart JN (2003) KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol 285:H921–H930
Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM (2005) Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288:H971–H976
Hausenloy DJ, Yellon DM (2004) New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res 61:448–460
Hausenloy DJ, Yellon DM (2006) Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res 70:240–253
Heidkamp MC, Bayer AL, Martin JL, Samarel AM (2001) Differential activation of mitogen-activated protein kinase cascades and apoptosis by protein kinase C epsilon and delta in neonatal rat ventricular myocytes. Circ Res 89:882–890
Hosoki R, Matsuki N, Kimura H (1997) The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237:527–531
Lebuffe G, Schumacker PT, Shao ZH, Anderson T, Iwase H, Vanden Hoek TL (2003) ROS and NO trigger early preconditioning: relationship to mitochondrial KATP channel. Am J Physiol Heart Circ Physiol 284:H299–H308
Liang BT (1998) Protein kinase C-dependent activation of KATP channel enhances adenosine-induced cardioprotection. Biochem J 336(Pt 2):337–343
Light PE, Kanji HD, Fox JE, French RJ (2001) Distinct myoprotective roles of cardiac sarcolemmal and mitochondrial KATP channels during metabolic inhibition and recovery. Faseb J 15:2586–2594
Maulik N, Watanabe M, Zu YL, Huang CK, Cordis GA, Schley JA, Das DK (1996) Ischemic preconditioning triggers the activation of MAP kinases and MAPKAP kinase 2 in rat hearts. FEBS Lett 396:233–237
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124–1136
Naitoh K, Ichikawa Y, Miura T, Nakamura Y, Miki T, Ikeda Y, Kobayashi H, Nishihara M, Ohori K, Shimamoto K (2006) MitoK(ATP) channel activation suppresses gap junction permeability in the ischemic myocardium by an ERK-dependent mechanism. Cardiovasc Res 70:374–383
Oh GS, Pae HO, Lee BS, Kim BN, Kim JM, Kim HR, Jeon SB, Jeon WK, Chae HJ, Chung HT (2006) Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med 41:106–119
Oldenburg O, Cohen MV, Yellon DM, Downey JM (2002) Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res 55:429–437
Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS (2006) Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol 40:119–130
Ping P, Zhang J, Cao X, Li RC, Kong D, Tang XL, Qiu Y, Manchikalapudi S, Auchampach JA, Black RG, Bolli R (1999) PKC-dependent activation of p44/p42 MAPKs during myocardial ischemia-reperfusion in conscious rabbits. Am J Physiol 276:H1468–H1481
Sivarajah A, McDonald MC, Thiemermann C (2006) The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 26:154–161
Toma O, Weber NC, Wolter JI, Obal D, Preckel B, Schlack W (2004) Desflurane preconditioning induces time-dependent activation of protein kinase C epsilon and extracellular signal-regulated kinase 1 and 2 in the rat heart in vivo. Anesthesiology 101:1372–1380
Yang G, Sun X, Wang R (2004) Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. Faseb J 18:1782–1784
Yellon DM, Downey JM (2003) Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83:1113–1151
Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH (2000) Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res 86:692–699
Zhao W, Ndisang JF, Wang R (2003a) Modulation of endogenous production of H2S in rat tissues. Can J Physiol Pharmacol 81:848–853
Zhao W, Zhang J, Lu Y, Wang R (2001) The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20:6008–6016
Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J (2003b) Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285:H579–H588
Wu S, Li HY, Wong TM (1999) Cardioprotection of preconditioning by metabolic inhibition in the rat ventricular myocyte. Involvement of κ-opioid receptor. Circ Res 84:1388–1395
Acknowledgments
The authors thank National University of Singapore, Office of Life Sciences (R184000074712) and National Medical Research Council grant (R184000132213) for their generous research support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, Y., Chen, X., Pan, TT. et al. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch - Eur J Physiol 455, 607–616 (2008). https://doi.org/10.1007/s00424-007-0321-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0321-4