Abstract
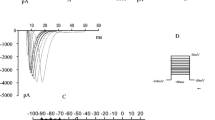
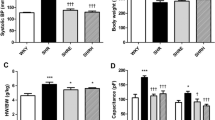
Inhibition of endothelin-A (ETA) receptors has been shown to reduce ventricular electrical abnormalities associated with cardiac failure. In this study, we investigate the effect of ETA-receptor inhibition on the development of regional alterations of the transient outward K+ current (I to) in the setting of pressure-induced left ventricular (LV) hypertrophy. Cardiac hypertrophy was induced in female Sprague–Dawley rats by stenosis of the ascending aorta (AS) for 7 days. Treatment with the selective ETA-receptor antagonist darusentan (LU135252, 35 mg [kg body weight]−1 day−1) was started 1 day before the surgery. AS induced a 46% increase in the relative LV weight (p < 0.001) and caused a significant reduction in I to (at +40 mV) in epicardial myocytes (19.5 ± 1.2 pA pF−1, n = 32 vs 23.2 ± 1.2 pA pF−1, n = 35, p < 0.05). Darusentan further reduced I to in AS (15.4 ± 1.3 pA pF−1, n = 37, p < 0.05) and sham-operated animals (19.8 ± 1.6 pA pF−1, n = 48, ns.). The effects of AS and darusentan on I to were significant and independent as tested by two-way analysis of variance. I to was not affected in endocardial myocytes. These results indicate that endothelin-1 may exert a tonic effect on the magnitude of I to in the epicardial region of the left ventricle but that ETA-receptor activation is not necessary for the development of electrical alterations associated with pressure-induced hypertrophy.




Similar content being viewed by others
References
Volk T, Nguyen THD, Schultz JH, Ehmke H (1999) Relationship between transient outward K+ current and Ca2+ influx in rat cardiac myocytes of endo- and epicardial origin. J Physiol 519:841–850
Sah R, Ramirez RJ, Backx PH (2002) Modulation of Ca2+ release in cardiac myocytes by changes in repolarization rate: role of phase-1 action potential repolarization in excitation–contraction coupling. Circ Res 90:165–173
Clark RB, Bouchard RA, Salinas SE, Sanchez CJ, Giles WR (1993) Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc Res 27:1795–1799
Volk T, Nguyen THD, Schultz JH, Faulhaber J, Ehmke H (2001) Regional alterations of repolarizing K+ currents among the left ventricular wall of rats with ascending aortic stenosis. J Physiol 530:443–455
Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH (2001) The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol 33:851–872
Zipes DP, Wellens HJ (1998) Sudden cardiac death. Circulation 98:2334–2351
Bryant SM, Shipsey SJ, Hart G (1999) Normal regional distribution of membrane current density in rat left ventricle is altered in catecholamine-induced hypertrophy. Cardiovasc Res 42:391–401
Guo W, Kamiya K, Hojo M, Kodama I, Toyama J (1998) Regulation of Kv4.2 and Kv1.4 K+ channel expression by myocardial hypertrophic factors in cultured newborn rat ventricular cells. J Mol Cell Cardiol 30:1449–1455
Yamauchi-Kohno R, Miyauchi T, Hoshino T, Kobayashi T, Aihara H, Sakai S, Yabana H, Goto K, Sugishita Y, Murata S (1999) Role of endothelin in deterioration of heart failure due to cardiomyopathy in hamsters: increase in endothelin-1 production in the heart and beneficial effect of endothelin-A receptor antagonist on survival and cardiac function. Circulation 99:2171–2176
Matsumoto Y, Aihara H, Yamauchi-Kohno R, Reien Y, Ogura T, Yabana H, Masuda Y, Sato T, Komuro I, Nakaya H (2002) Long-term endothelin a receptor blockade inhibits electrical remodeling in cardiomyopathic hamsters. Circulation 106:613–619
Wiesner RJ, Ehmke H, Faulhaber J, Zak R, Rüegg JC (1997) Dissociation of left ventricular hypertrophy, β-myosin heavy chain gene expression, and myosin isoform switch in rats after ascending aortic stenosis. Circulation 95:1253–1259
Moser L, Faulhaber J, Wiesner RJ, Ehmke H (2002) Predominant activation of endothelin-dependent cardiac hypertrophy by norepinephrine in rat left ventricle. Am J Physiol 282:R1389–R1394
Wiesner RJ, Aschenbrenner V, Rüegg JC, Zak R (1994) Coordination of nuclear and mitochondrial gene expression during the development of cardiac hypertrophy in rats. Am J Physiol 267:C229–C235
Volk T, Noble PJ, Wagner M, Noble D, Ehmke H (2005) Ascending aortic stenosis selectively increases action potential-induced Ca2+ influx in epicardial myocytes of the rat left ventricle. Exp Physiol 90:111–121
Volk T, Ehmke H (2002) Conservation of L-type Ca2+ current characteristics in endo- and epicardial myocytes from rat left ventricle with pressure-induced hypertrophy. Pflügers Arch 443:399–404
Isenberg G, Klöckner U (1982) Calcium tolerant ventricular myocytes prepared by preincubation in a “KB medium”. Pflügers Arch 395:6–18
Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ (1997) Defective excitation–contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276:800–806
Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, Rosen MR, Cohen IS (2000) Effects of the renin–angiotensin system on the current Ito in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res 86:1062–1068
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Rothermund L, Vetter R, Dieterich M, Kossmehl P, Gogebakan O, Yagil C, Yagil Y, Kreutz R (2002) Endothelin-A receptor blockade prevents left ventricular hypertrophy and dysfunction in salt-sensitive experimental hypertension. Circulation 106:2305–2308
Luscher TF, Enseleit F, Pacher R, Mitrovic V, Schulze MR, Willenbrock R, Dietz R, Rousson V, Hurlimann D, Philipp S, Notter T, Noll G, Ruschitzka F (2002) Hemodynamic and neurohumoral effects of selective endothelin A (ETA) receptor blockade in chronic heart failure: the Heart Failure ETA Receptor Blockade Trial (HEAT). Circulation 106:2666–2672
Xia QG, Reinecke A, Dorenkamp M, Daemen MJ, Simon R, Unger T (2006) Effects of endothelin ETA receptor blocker LU 135252 on cardiac remodeling and survival in a hypertensive rat model of chronic heart failure. Acta Pharmacol Sin 27:1417–1422
Hafizi S, Wharton J, Chester AH, Yacoub MH (2004) Profibrotic effects of endothelin-1 via the ETA receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem 14:285–292
Anversa P, Leri A, Rota M, Bearzi C, Urbanek K, Kajstura J, Bolli R (2006) Stem cells, myocardial regeneration and methodological artifacts. Stem Cells (in press). DOI 10.1634/stemcells.2006-0623
Brutsaert DL (2003) Cardiac endothelial–myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83:59–115
Brown RD, Ambler SK, Mitchell MD, Long CS (2005) The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 45:657–687
Berthold H, Münter K, Just A, Kirchheim HR, Ehmke H (1999) Stimulation of the renin-angiotensin system by endothelin subtype. A receptor blockade in conscious dogs. Hypertension 33:1420–1424
Himmel HM, Wettwer E, Li Q, Ravens U (1999) Four different components contribute to outward current in rat ventricular myocytes. Am J Physiol 277:H107–H118
Acknowledgments
We are most grateful to Telse Kock for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagner, M., Goltz, D., Stucke, C. et al. Modulation of the transient outward K+ current by inhibition of endothelin-A receptors in normal and hypertrophied rat hearts. Pflugers Arch - Eur J Physiol 454, 595–604 (2007). https://doi.org/10.1007/s00424-007-0227-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-007-0227-1