Abstract
For single neuron models, reproducing characteristics of neuronal activity such as the firing rate, amplitude of spikes, and threshold potentials as functions of both synaptic current and conductance is a challenging task. In the present work, we measure these characteristics of regular spiking cortical neurons using the dynamic patch-clamp technique, compare the data with predictions from the standard Hodgkin-Huxley and Izhikevich models, and propose a relatively simple five-dimensional dynamical system model, based on threshold criteria. The model contains a single sodium channel with slow inactivation, fast activation and moderate deactivation, as well as, two fast repolarizing and slow shunting potassium channels. The model quantitatively reproduces characteristics of steady-state activity that are typical for a cortical pyramidal neuron, namely firing rate not exceeding 30 Hz; critical values of the stimulating current and conductance which induce the depolarization block not exceeding 80 mV and 3, respectively (both values are scaled by the resting input conductance); extremum of hyperpolarization close to the midpoint between spikes. The analysis of the model reveals that the spiking regime appears through a saddle-node-on-invariant-circle bifurcation, and the depolarization block is reached through a saddle-node bifurcation of cycles. The model can be used for realistic network simulations, and it can also be implemented within the so-called mean-field, refractory density framework.









Similar content being viewed by others
Notes
Available at: http://www.ioffe.ru/CompPhysLab/AntonV3.html
References
Alzheimer C, Schwindt PC, Crill WE (1993) Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci 13:660–673
Amakhin DV, Soboleva EB, Postnikova TYu, Tumanova NL, Dubrovskaya NM, Kalinina DS, Vasiliev DS, Zaitsev AV (2022) Maternal Hypoxia Increases the Excitability of Neurons in the Entorhinal Cortex and Dorsal Hippocampus of Rat Offspring // Frontiers in Neuroscience 16:867120. DOI: https://doi.org/10.3389/fnins.2022.867120.
Berndt A, Lee SY, Ramakrishnan C, Deisseroth K (2014) Structure-Guided Transformation of Channelrhodopsin into a Light-Activated Chloride Channel. Science 344:420-424
Bianchi D, Marasco A, Limongiello A, Marchetti C, Marie H, Tirozzi B, Migliore MJ (2012) On the mechanisms underlying the depolarization block in the spiking dynamics of CA1 pyramidal neurons. Comput Neurosci 33(2):207–225
Borg-Graham LJ, Monier C, Fregnac Y (2003) Visual input evokes transient and strong shunting inhibition in visual cortical neurons. Nature 393(6683):369–373
Brette R, Gerstner W (2005) Adaptive exponential integrate-and-firemodel as an effective description of neuronal activity. J Neurophysiol 94(5):3637–3642
Chen W, Zhang JJ, Hu GY, Wu CP (1996) Different mechanisms underlying the repolarization of narrow and wide action potentials in pyramidal cells and interneurons of cat motor cortex. Neuroscience 73(1):57–68
Chizhov AV (2017) Conductance-based refractory density approach: comparison with experimental data and generalization to lognormal distribution of input current. Biol Cybernetics 111(5–6):353–364
Chizhov AV, Graham LJ (2007) Population model of hippocampal pyramidal neurons, linking a refractory density approach to conductance-based neurons. Phys Rev E 75:011924
Chizhov AV, Graham LJ (2021) A strategy for mapping biophysical to abstract neuronal network models applied to primary visual cortex. PLoS Comput Biol 17(8):e1009007. https://doi.org/10.1371/journal.pcbi.1009007
Chizhov AV, Yu SE, Kh KK, Zaitsev AV (2014) A simple Markov model of sodium channels with a dynamic threshold. J Comp Neuroscience 37(1):181–191
Chizhov AV, Amakhin DV, Zaitsev AV (2019) Mathematical model of Na-K-Cl homeostasis in ictal and interictal discharges. PLoS ONE 14(3):e0213904
Cressman JR, Ullah G, Ziburkus J, Schiff SJ, Barreto E (2009) The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single Neuron Dynamics J Comput Neurosci 26(2):159–170
Destexhe A, Bal T (2009) Dynamic clamp: from principles to applications. Springer
Dovzhenok A, Kuznetsov AS (2012) Exploring neuronal bistability at the depolarization block. PLoS ONE 7(8):e42811
Ergina JL, Amakhin DV, Postnikova TY, Soboleva EB, Zaitsev AV (2021) Short-term epileptiform activity potentiates excitatory synapses but does not affect intrinsic membrane properties of pyramidal neurons in the rat hippocampus in vitro. Biomedicines 10(9):1374
Fernandez FR, White JA (2009) Reduction of spike after depolarization by increased leak conductance alters interspike interval variability. J Neurosc 29(4):973–986
Fernandez FR, White JA (2010) Gain control in CA1 pyramidal cells using changes in somatic conductance. J Neurosc 30(1):230–241
Fernandez FR, Broicher T, Truong A, White JA (2011) Membrane voltage fluctuations reduce spike frequency adaptation and preserve output gain in CA1 pyramidal neurons in a high conductance state. J Neurosci 31(10):3880–3893
Gerstner W, Naud R (2009) How good are neuron models? Science 326(5951):379–380
Górski T, Depannemaecker D, Destexhe A (2021) Conductance-based adaptive exponential integrate-and-fire model. Neural Comput 33:41–66
Graham LJ, Schramm A (2009) In vivo dynamic clamp: The functional impact of synaptic and intrinsic conductances in visual cortex. In Destexhe A, & Bal T (Eds.), Dynamic clamp: From principles to applications. Springer Press, (2009).
Gutkin B, Ermentrout GB (2006) Spikes too kinky in the cortex? Nature 440(7087):999–1000
Hamill OP, Huguenard JR, Prince DA (1991) Patch-clamp studies of voltage-gated currents in identified neurons of the rat cerebral cortex. Cereb Cortex 1:48–61
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500–544
Izhikevich EM (2003) Simple model of spiking neurons. IEEE Trans Neural Networks 14:1569–1572
Krishnan GP, Bazhenov M (2011) Ionic dynamics mediate spontaneous termination of seizures and postictal depression state. J Neurosci 31(24):8870–8882
Mainen ZF, Sejnowski TJ (1996) Influence of dendritic structure on firing pattern in model neocortical neurons. Nature 382:363–366
McCormick DA, Shu Y, Yu Y (2007) Neurophysiology: Hodgkin and Huxley model–still standing? Nature 445:E1–E2
Naundorf B, Wolf F, Volgushev M (2006) Unique features of action potential initiation in cortical neurons. Nature 440(7087):1060–1063
Pinsky PF, Rinzel J (1994) Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. J Comput Neurosci 1(1–2):39–60
Platkiewicz J, Brette R (2010) A threshold equation for action potential initiation. PLoS Comput Biol 6(7):e1000850
Pokrovskii AN (1978) Effect of synapse conductivity on spike development. Biofizika 23(4):649–653
Pospischil M, Monier C, Piwkowska Z, Bal T, Frégnac Y, Destexhe A (2008) Minimal Hodgkin-Huxley type models for different classes of cortical and thalamic neurons. Biol Cybern 99:427–441
Prinz AA, Abbott LF, Marder E (2004) The dynamic clamp comes of age. Trends Neurosci 27:218–224
Qian K, Yu N, Tucker KR, Levitan ES, Canavier CC (2014) Mathematical analysis of depolarization block mediated by slow inactivation of fast sodium channels in midbrain dopamine neurons. J Neurophysiol 112(11):2779–2790
Rinzel J, Baer SM (1988) Threshold for repetitive activity for a slow stimulus ramp: a memory effect and its dependence on fluctuations. Biophys J 54(3):551–555
Schwalger T, Chizhov A (2019) Mind the last spike–firing rate models for mesoscopic populations of spiking neurons. Curr Opinion in Neurobiology 58:155–166
Shao L-R, Halvorsrud R, Borg-GrahamStorm LJF (1999) The role of BKtype Cadependent K-channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521(1):135–146
Shriki O, Hansel D, Sompolinsky H (2003) Rate models for conductance-based cortical neuronal networks. Neural Comput 15(8):1809–1841
Smirnova EY, Zaitsev AV, Kim KKh, Chizhov AV (2015) The domain of neuronal firing on a plane of input current and conductance. J Comput Neurosci 39(2):217–233
Stafstrom CE (2007) Persistent sodium current and its role in epilepsy. Epilepsy Curr 7(1):15–22
Tucker KR, Huertas MA, Horn JP, Canavier CC, Levitan ES (2012) Pacemaker rate and depolarization block in nigral dopamine neurons: a somatic sodium channel balancing act. J Neurosci 32(42):14519–14531
Vervaeke K, Hu H, Graham LJ, Storm JF (2006) Contrasting effects of the persistent Na+ current on neuronal excitability and spike timing. Neuron 49:257–270
Wei Y, Ullah G, Ingram J, Schiff SJ (2014) Oxygen and seizure dynamics: II. Computational modeling. J Neurophysiol 112:213–223
Funding
This work was supported by the Russian Science Foundation (project 21-15-00416) for D.A.
Author information
Authors and Affiliations
Contributions
A.C. and D.M. formulated the problem. D.A. performed the experiments. A.C. wrote software for experiments. A.C. and E.S. performed simulations. M.D. provided the bifurcation analysis. A.C., D.A. and M.D. wrote the main manuscript text.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Additional information
Communicated by Benjamin Lindner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix
Experimental technique
2.1 The whole-cell patch-clamp recordings in rat brain slices
Animal handling and experimentation were performed in accordance with European Community Council directives 86/609/EEC. Male Wistar albino rats were used for the experiments (N = 4, 18–21 days postnatal). Rats were sacrificed by decapitation, each brain was rapidly removed and immersed in ice-cold, preoxygenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 126 NaCl, 2.5 KCl, 1.25 Na2HPO4, 24 NaHCO3, 2 CaCl2, 1 MgSO4, 10 D-glucose; pH 7.3–7.4. Coronal slices (300 μm thick) of medial frontal (prelimbic) cortex were cut with a vibratome (Microm HM 650 V; Microm, Walldorf, Germany). Slices were incubated at room temperature for at least 1 h before recordings. Neurons were visualized using transmitted illumination on a fixed-stage upright Axioscop 2 microscope (Zeiss, Oberkochen, Germany) equipped with differential interference contrast optics and a video camera (Grasshopper 3 GS3-U3-23S6M-C; FLIR Integrated Imaging Solutions Inc., Wilsonville, OR, USA). Pyramidal neurons were identified by their apical dendrites and triangular somata. Whole-cell recordings were made from layer 3 pyramidal neurons using Model 2400 (AM-Systems; Sequim, WA, USA) patch-clamp amplifier. Patch electrodes (3–5 MΩ) were pulled from borosilicate capillary glass (Sutter Instrument, Novato, CA, USA). The following internal solution was used (in mM): 136 K-gluconate, 10 NaCl, 5 EGTA, 10 HEPES, 4 ATP-Mg, 0.3 GTP, pH 7.25 (adjusted with KOH).
2.2 Acquisition and dynamic-clamp
Signals were digitized with a sampling frequency of 33 kHz for acquisition with NI DAQ PCI-6221-37pin (National Instruments, Austin, TX) and filtered with 5 kHz by the amplifier. Whole-cell recordings were done in the dynamic-clamp mode to introduce additional leaky channel. The custom software “DC-project” was used.Footnote 1 The applied current is calculated as \(u(t)-s(t)(V(t)-{V}_{US})\), where \(u(t)\) and \(s(t)\) are the voltage-independent input signals, current and conductance, respectively; \(V(t)\) is the membrane voltage and \({V}_{US}\) is the reference voltage which was set to -60 mV, close to the resting membrane potential \({V}_{\mathrm{rest}}\). This reference voltage level is not essential, because its shift is equivalent to the shift of u. We included it for analysis only cells with stable input conductance and membrane potential, and with high access conductance. The access resistance was compensated in real time. For every neuron the recordings were started from estimations of the resting membrane potential \({V}_{\mathrm{rest}}\), input conductance \({G}_{L}\), and the membrane time constant \({\tau }_{m}\). Intrinsic membrane properties were assessed from the voltage responses to the series of 500-ms current steps providing hyperpolarization up to 5–10 mV.
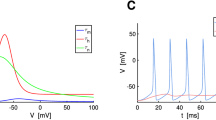
To estimate the f-u-s-function in the whole domain of u and s that provide spike generation, a series of recordings were performed with the injected steps of input signal calculated for different values of current \(u\) and conductance \(s\) and lasting for 0.5 s with the frequency 0.5 Hz. The increments of u and s were constant, the typical grid was 25 by 12 for u and s, respectively. For each step of stimulation, the firing rate ν was calculated as a total number of spikes per the last 2/3 of step duration. The values of u and s in the plots were scaled by the input conductance \({G}_{L}\), in order to compare Ω-domains for different neurons, following (Graham and Schramm 2009).
The threshold was measured at the point where \(\mathrm{d}V/\mathrm{d}t\) reaches \(5 \mathrm{mV}/\mathrm{ms}\) or \({\mathrm{d}}^{2}V/\mathrm{d}{t}^{2}\) reaches \(2 \mathrm{mV}/{\mathrm{ms}}^{2}\). The spike half-width was measured for each spike at the half-height defined as the middle voltage level between the threshold and the peak, and averaged across all spikes of a trace. The post-spike hyperpolarization potential (PHP) was measured as a minimum value between spikes, and averages across spikes. The average membrane potential was calculated for each trace on interspike intervals since the time moment of the peak plus 2 ms to the time of the next crossing of threshold.
Mathematical models
3.1 Hodgkin–Huxley model
We considered the classical one-compartmental Hodgkin-Huxley model, described by the following equations (Hodgkin and Huxley 1952):
where \(U(t)\) is the membrane potential, \(C\) is the capacitance, \({V}_{L}, {V}_{\mathrm{Na}}\) and \({V}_{K}\) are the leak, sodium and potassium reversal potentials, \(m(U,t)\) is the sodium channel activation, \(h(U,t)\) is the sodium channel activation, \(n(U,t)\) is the potessium channel activation, \({g}_{L}\) is the leak conductance, \({\overline{g}}_{\mathrm{Na}} \) and \({\overline{g}}_{K}\) are the sodium and potassium channel maximum conductances.
Here, in comparison with standard parameterization, the leak reversal potential \({V}_{L}\) was modified to provide the desired resting potential \({V}_{\mathrm{rest}}\), and the membrane area was set such that the input conductance at rest, \({G}_{L}\), would be equal to 5nS as in the other model.
3.2 Izhikevich model
The simple model of a regular spiking cell was used according to (Izhikevich 2003), where to we introduced the input terms \(u\left(t\right)-s\left(t\right)\left(U-{V}_{us}\right)\):
with the after-spike resetting.
if \(U\ge 30\, \mathrm{mV}\), then \(U=c\), \(w=w+d\).
The parameters were normalized so that the neuron has an input conductance of 5nS, namely: \(C=\left(100*\frac{5}{35}\right)\, \mathrm{pF}\), \(k=\left(3*\frac{5}{35}\right)\,\mathrm{nS}/\mathrm{mV}\), \({V}_{r}=-60 \,\mathrm{mV}\), \({V}_{t}=-50\, \mathrm{mV}\), \(a=\left(0.01*\frac{35}{5}\right) \,{\mathrm{ms}}^{-1}\), \(b=\left(5*\frac{5}{35}\right)\,\mathrm{nS}\), \(c=-60 \,\mathrm{mV}\), \(d=400\, \mathrm{pA}\).
3.3 Minimal Hodgkin–Huxley-like model for rodent’s cortical regular spiking neuron
The model is taken from (Pospischil et al. 2008). A slow potassium channel with the activalion variable \(p(U,t)\) was added to the original Hodgkin–Huxley model:
3.4 The proposed hybrid model
The proposed model contains three channels: one sodium channel \({I}_{\mathrm{Na}}\) and two potassium channels, one fast \({I}_{K,f}\) and one slow \({I}_{K,s}\). The voltage equation hence reads:
The sodium current is defined by:
\({i}_{\infty }=1/(1+\mathrm{exp} ((U+44)/4)),\) Hence \({i}_{\infty }^{-1}\left(x\right)=-44+4\, \mathrm{ln}\left(1/x-1\right)\)
The fast voltage-dependent potassium current \({I}_{K,f}\) is defined by:
The slow voltage-dependent potassium current \({I}_{K,s}\) is defined by:
Parameters values are given by:
For a fast-spiking interneuron, not considered in the present paper, we modified two parameters: \({\overline{g}}_{K,s}=0.1\frac{\mu S}{{\mathrm{cm}}^{2}}\) and \({\tau }_{mm}=3 \mathrm{ms}\), which increases the gain of the f-I curve, increases the maximum firing rate and approaches the DB limit.
3.5 The proposed continuous model
A continuous version of the sodium channel approximation is obtained from the system of Eqs. (1–11) by omitting the threshold condition, Eq. (3), and introducing in Eqs. (5, 6) the variable time constants and steady-state functions in the following form:
3.5.1 Statistical analysis
For each spike, the threshold potential in the model was measured at 1.2 ms before the peak of the spike. The spike half-width was measured for each spike at the voltage level − 20 mV, and averaged across all spikes of a trace. The post-spike hyperpolarization potential (PHP) was measured as a minimum value between spikes, and averages across spikes. The average membrane potential was calculated for each trace on interspike intervals since the time moment of the peak plus 2 ms to the point where \(\mathrm{d}V/\mathrm{d}t\) reaches \(5 \mathrm{mV}/\mathrm{ms}\).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chizhov, A.V., Amakhin, D.V., Sagtekin, A.E. et al. Single-compartment model of a pyramidal neuron, fitted to recordings with current and conductance injection. Biol Cybern 117, 433–451 (2023). https://doi.org/10.1007/s00422-023-00976-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-023-00976-7