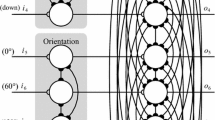
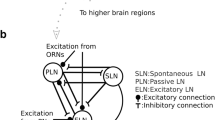
Abstract
Visual binding is the process of associating the responses of visual interneurons in different visual submodalities all of which are responding to the same object in the visual field. Recently identified neuropils in the insect brain termed optic glomeruli reside just downstream of the optic lobes and have an internal organization that could support visual binding. Working from anatomical similarities between optic and olfactory glomeruli, we have developed a model of visual binding based on common temporal fluctuations among signals of independent visual submodalities. Here we describe and demonstrate a neural network model capable both of refining selectivity of visual information in a given visual submodality, and of associating visual signals produced by different objects in the visual field by developing inhibitory neural synaptic weights representing the visual scene. We also show that this model is consistent with initial physiological data from optic glomeruli. Further, we discuss how this neural network model may be implemented in optic glomeruli at a neuronal level.












Similar content being viewed by others
References
Adelson EH, Bergen JR (1985) Spatiotemporal energy models for the perception of motion. J Opt Soc Am A 2:284–299
Albrecht DG, Geisler WS (1991) Motion selectivity and the contrast-response function of simple cells in the visual cortex. Vis Neurosci 7(6):531–546
Anderson JA (1995) An introduction to neural networks. MIT Press, Cambridge
Arnett DW (1972) Spatial and temporal integration properties of units in first optic ganglion of dipterans. J Neurophysiol 35(4):429–444
Barlow HB (2001) Redundancy reduction revisited. Netw Comp Neural 12(3):241–253
Bazhenov M, Stopfer M, Rabinovich M, Abarbanel HD, Sejnowski TJ, Laurent G (2001) Model of cellular and network mechanisms for odor-evoked temporal patterning in the locust antennal lobe. Neuron 30(2):569–581
Bi GQ, Poo MM (1998) Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 18(24):10,464–10,472
Borst A, Egelhaaf M (1989) Principles of visual motion detection. Trends Neurosci 12(8):297–306
Carandini M, Heeger DJ (2012) Normalization as a canonical neural computation. Nat Rev Neurosci 13(1):51–62
Cichocki A, Bogner RE, Moszczyński L, Pope K (1997) Modified Herault–Jutten algorithms for blind separation of sources. Digit Signal Process 7(2):80–93
Crick F, Koch C (1990) Towards a neurobiological theory of consciousness. Semin Neurosci 2:263–275
Douglass JK, Strausfeld NJ (2005) Sign-conserving amacrine neurons in the fly’s external plexiform layer. Vis Neurosci 22(03):345–358
Eckhorn R, Reitboeck HJ, Arndt M, Dicke P (1990) Feature linking via synchronization among distributed assemblies: simulations of results from cat visual cortex. Neural Comput 2(3):293–307
Engel AK, Singer W (2001) Temporal binding and the neural correlates of sensory awareness. Trends Cognit Sci 5(1):16–25
Engel AK, König P, Kreiter AK, Schillen TB, Singer W (1992) Temporal coding in the visual cortex: new vistas on integration in the nervous system. Trends Neurosci 15(6):218–226
Fonta C, Sun XJ, Masson C (1993) Morphology and spatial distribution of bee antennal lobe interneurones responsive to odours. Chem Senses 18(2):101–119
Gerstner W, Kistler WM (2002) Mathematical formulations of Hebbian learning. Biol Cybern 87(5–6):404–415
Gerstner W, Kempter R, van Hemmen JL, Wagner H (1996) A neuronal learning rule for sub-millisecond temporal coding. Nature 383(6595):76
Hassenstein B, Reichardt W (1956) Systemtheoretische Analyse der Zeit-, Reihenfolgen- und Vorzeichenauswertung bei der Bewegungsperzeption des Rüsselkäfers Chlorophanus. Z Naturforsch B 11(9–10):513–524
Hebb DO (1949) The organization of behavior: a neuropsychological theory. Wiley, New York
Heisenberg M (2003) Mushroom body memoir: From maps to models. Nat Rev Neurosci 4(4):266–275
Herault J, Jutten C (1986) Space or time adaptive signal processing by neural network models. In: AIP Conference Proceedings, Snowbird, vol 151. American Institute of Physics, pp 206–211
Hildebrand JG (1996) Olfactory control of behavior in moths: central processing of odor information and the functional significance of olfactory glomeruli. J Comp Physiol A 178(1):5–19
Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: Converging evidence for common principles across phyla. Annu Rev Neurosci 20(1):595–631
Hopfield JJ (1991) Olfactory computation and object perception. Proc Natl Acad Sci USA 88(15):6462–6466
Hubel DH, Wiesel TN (1959) Receptive fields of single neurones in the cat’s striate cortex. J Physiol 148(3):574–591
Hubel DH, Wiesel TN (1968) Receptive fields and functional architecture of monkey striate cortex. J Physiol 195(1):215–243
Hummel JE, Biederman I (1992) Dynamic binding in a neural network for shape recognition. Psychol Rev 99(3):480–517
Hyvärinen A, Oja E (1998) Independent component analysis by general nonlinear Hebbian-like learning rules. Signal Process 64(3):301–313
Itti L, Koch C, Niebur E (1998) A model of saliency-based visual attention for rapid scene analysis. IEEE Trans Pattern Anal 11:1254–1259
Jefferis GSXE (2005) Insect olfaction: a map of smell in the brain. Curr Biol 15(17):R668–R670
Joho M, Mathis H, Lambert RH (2000) Overdetermined blind source separation: Using more sensors than source signals in a noisy mixture. In: Proc. International Conference on Independent Component Analysis and Blind Signal Separation. Helsinki, pp 81–86
Jutten C, Herault J (1991) Blind separation of sources, part I: an adaptive algorithm based on neuromimetic architecture. Signal Process 24(1):1–10
Koch C (1999) Biophysics of computation: information processing in single neurons. Oxford University Press, New York
Land MF, Nilsson DE (2002) Animal eyes. Oxford University Press, New York
Laughlin S (1983) Matching coding to scenes to enhance efficiency. Proceedings of an International Symposium Organized by The Rank Prize Funds, Springer Series in Information Sciences. Springer, London, pp 42–52
Linster C, Masson C (1996) A neural model of olfactory sensory memory in the honeybee’s antennal lobe. Neural Comput 8(1):94–114
Linster C, Smith BH (1997) A computational model of the response of honey bee antennal lobe circuitry to odor mixtures: overshadowing, blocking and unblocking can arise from lateral inhibition. Behav Brain Res 87(1):1–14
von der Malsburg C (1994) The correlation theory of brain function. Springer, New York
von der Malsburg C (1999) The what and why of binding: the modeler’s perspective. Neuron 24(1):95–104
Markram H, Lübke J, Frotscher M, Sakmann B (1997) Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275(5297):213–215
Martin AB, von der Heydt R (2015) Spike synchrony reveals emergence of proto-objects in visual cortex. J Neurosci 35(17):6860–6870
Mu L, Ito K, Bacon JP, Strausfeld NJ (2012) Optic glomeruli and their inputs in Drosophila share an organizational ground pattern with the antennal lobes. J Neurosci 32(18):6061–6071
Nakayama K, Silverman GH (1988) The aperture problem—I. Perception of nonrigidity and motion direction in translating sinusoidal lines. Vis Res 28(6):739–746
Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenböck G (2002) Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36(3):463–474
Northcutt BD, Higgins CM (2017) An insect-inspired model for visual binding II: Functional analysis and visual attention. Biol Cybern. doi:10.1007/s00422-017-0716-z
Okamura JY, Strausfeld NJ (2007) Visual system of calliphorid flies: motion- and orientation-sensitive visual interneurons supplying dorsal optic glomeruli. J Comp Neurol 500(1):189–208
Paulk AC, Dacks AM, Phillips-Portillo J, Fellous JM, Gronenberg W (2009) Visual processing in the central bee brain. J Neurosci 29(32):9987–9999
Rivera-Alvidrez Z, Lin I, Higgins CM (2011) A neuronally based model of contrast gain adaptation in fly motion vision. Vis Neurosci 28(5):419–431
Rodieck RW (1965) Quantitative analysis of cat retinal ganglion cell response to visual stimuli. Vis Res 5(12):583–601
van Santen JPH, Sperling G (1985) Elaborated Reichardt detectors. J Opt Soc Am A 2(5):300–320
Schillen TB, König P (1994) Binding by temporal structure in multiple feature domains of an oscillatory neuronal network. Biol Cybern 70(5):397–405
Schwartz O, Simoncelli EP (2001) Natural signal statistics and sensory gain control. Nat Neurosci 4(8):819–825
Simoncelli EP, Olshausen BA (2001) Natural image statistics and neural representation. Annu Rev Neurosci 24(1):1193–1216
Snyder AW (1979) Physics of vision in compound eyes. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6A. Springer, Berlin, pp 225–313 (chap 5)
Song S, Miller KD, Abbott LF (2000) Competitive Hebbian learning through spike-timing-dependent synaptic plasticity. Nat Neurosci 3(9):919–926
Srinivasan MV, Zhang SW, Witney K (1994) Visual discrimination of pattern orientation by honeybees: performance and implications for ‘cortical’ processing. Philos Trans R Soc B 343(1304):199–210
Stavenga DG (1979) Pseudopupils of compound eyes. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6A. Springer, Berlin, pp 357–439 (chap 7)
Strausfeld NJ, Okamura JY (2007) Visual system of calliphorid flies: organization of optic glomeruli and their lobula complex efferents. J Comp Neurol 500(1):166–188
Strausfeld NJ, Sinakevitch I, Okamura JY (2007) Organization of local interneurons in optic glomeruli of the dipterous visual system and comparisons with the antennal lobes. Dev Neurobiol 67(10):1267–1288
Trentelman H, Stoorvogel AA, Hautus M (2012) Control theory for linear systems. Springer Science & Business Media, Berlin
Yang EC, Maddess T (1997) Orientation-sensitive neurons in the brain of the honey bee (Apis mellifera). J Insect Physiol 43(4):329–336
Acknowledgements
The authors would like to thank the Air Force Office of Scientific Research for early support of this project with Grant Number FA9550-07-1-0165, and the Air Force Research Laboratories for supporting this research to maturity with STTR Phase I Award Number FA8651-13-M-0085 and Phase II Award Number FA8651-14-C-0108, both in collaboration with Spectral Imaging Laboratory (Pasadena, CA). We would also like to thank the reviewers, whose input greatly enhanced this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Northcutt, B.D., Dyhr, J.P. & Higgins, C.M. An insect-inspired model for visual binding I: learning objects and their characteristics. Biol Cybern 111, 185–206 (2017). https://doi.org/10.1007/s00422-017-0715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-017-0715-0