Abstract
Purpose
Mitochondrial dynamics are regulated by the differing molecular pathways variously governing biogenesis, fission, fusion, and mitophagy. Adaptations in mitochondrial morphology are central in driving the improvements in mitochondrial bioenergetics following exercise training. However, there is a limited understanding of mitochondrial dynamics in response to inactivity.
Methods
Skeletal muscle biopsies were obtained from middle-aged males (n = 24, 49.4 ± 3.2 years) who underwent sequential 14-day interventions of unilateral leg immobilisation, ambulatory recovery, and resistance training. We quantified vastus lateralis gene and protein expression of key proteins involved in mitochondrial biogenesis, fusion, fission, and turnover in at baseline and following each intervention.
Results
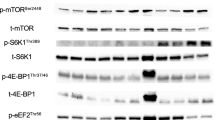
PGC1α mRNA decreased 40% following the immobilisation period, and was accompanied by a 56% reduction in MTFP1 mRNA, a factor involved in mitochondrial fission. Subtle mRNA decreases were also observed in TFAM (17%), DRP1 (15%), with contrasting increases in BNIP3L and PRKN following immobilisation. These changes in gene expression were not accompanied by changes in respective protein expression. Instead, we observed subtle decreases in NRF1 and MFN1 protein expression. Ambulatory recovery restored mRNA and protein expression to pre-intervention levels of all altered components, except for BNIP3L. Resistance training restored BNIP3L mRNA to pre-intervention levels, and further increased mRNA expression of OPA-1, MFN2, MTFP1, and PINK1 past baseline levels.
Conclusion
In healthy middle-aged males, 2 weeks of immobilisation did not induce dramatic differences in markers of mitochondria fission and autophagy. Restoration of ambulatory physical activity following the immobilisation period restored altered gene expression patterns to pre-intervention levels, with little evidence of further adaptation to resistance exercise training.




Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AIFM2:
-
Apoptosis-inducing factor 2
- AR:
-
Ambulatory recovery
- BL:
-
Baseline
- DRP1:
-
Dynamin-1-like protein
- Fis1:
-
Mitochondrial fission 1 protein
- IM:
-
Immobilisation
- LC3B:
-
MAP1 light chain 3
- MFF:
-
Mitochondrial fission factor
- MFN1:
-
Mitofusin 1
- MFN2:
-
Mitofusin 2
- MTFP1:
-
Mitochondrial fission process protein 1
- NRF1:
-
Nuclear regulatory factor 1
- NRF2:
-
Nuclear regulatory factor 2
- OPA1:
-
Optic atrophy 1
- PINK1:
-
PTEN-induced putative kinase 1
- PGC1α:
-
Peroxisome proliferator-activated receptor γ coactivator-1α
- ROS:
-
Reactive oxygen species
- RT:
-
Resistance training
- TFAM:
-
Mitochondrial transcription factor A
References
Abadi A, Glover EI, Isfort RJ et al (2009) Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One 4(8):e6518. https://doi.org/10.1371/journal.pone.0006518
Abramoff MD, Magalhaes PJ, Ram SJ (2004) Image Processing with ImageJ. Biophotonics Int 11:36–42
Adhihetty PJ, O’Leary MF, Chabi B et al (2007) Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol (1985) 102(3):1143–1151
Alibegovic AC, Sonne MP, Hojbjerre L et al (2010) Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Metab 299(5):E752–E763. https://doi.org/10.1152/ajpendo.00590.2009
Ashrafi G, Schwarz TL (2013) The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ 20(1):31–42. https://doi.org/10.1038/cdd.2012.81
Baar K, Wende AR, Jones TE et al (2002) Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 16:1879–1886. https://doi.org/10.1096/fj.02-0367com
Brandt N, Gunnarsson TP, Bangsbo J, Pilegaard H (2018) Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Physiol Rep 6(7):e13651. https://doi.org/10.1414/phy2.13651
Breen L, Stokes KA, Churchward-Venne TA et al (2013) Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98(6):2604–2612. https://doi.org/10.1210/jc.2013-1502
Byrne CA, McNeil AT, Koh TJ et al (2019) Expression of genes in the skeletal muscle of individuals with cachexia/sarcopenia: a systematic review. PLoS One 14(9):e0222345. https://doi.org/10.1371/journal.pone.0222345
Carter HN, Chen CC, Hood DA (2015) Mitochondria, muscle health, and exercise with advancing age. Physiology (bethesda) 30(3):208–223. https://doi.org/10.1152/physiol.00039.2014
Cereghetti GM, Stangherlin A, De Brito OM et al (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105(41):15803–15808. https://doi.org/10.1073/pnas.0808249105
Chan DC (2006) Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22:79–99. https://doi.org/10.1146/annurev.cellbio.22.010305.104638
Edwards SJ, Smeuninx B, Mckendry J et al (2020) High-dose leucine supplementation does not prevent muscle atrophy or strength loss over 7 days of immobilization in healthy young males. Am J Clin Nutr 112(5):1368–1381. https://doi.org/10.1093/ajcn/nqaa229
Elgass K, Pakay J, Ryan MT, Palmer CS (2013) Recent advances into the understanding of mitochondrial fission. Biochim Biophys Acta Mol Cell Res 133(1):150–161. https://doi.org/10.1016/j.bbamcr.2012.05.002
Glancy B, Hartnell LM, Malide D et al (2015) Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523(7562):617–620. https://doi.org/10.1038/nature14614
Goldspink G (1999) Changes in muscle mass and phenotype and the expression of autocrine and systemic growth factors by muscle in response to stretch and overload. J Anat 194:323–334. https://doi.org/10.1046/j.1469-7580.1999.19430323.x
Gomes LC, Di Benedetto G, Scorrano L (2011) During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13(5):589–598. https://doi.org/10.1038/ncb2220
Gram M, Vigelsø A, Yokota T et al (2014) Two weeks of one-leg immobilization decreases skeletal muscle respiratory capacity equally in young and elderly men. Exp Gerontol 58:269–278. https://doi.org/10.1016/j.exger.2014.08.013
Handschin C, Spiegelman BM (2006) Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27(7):728–735. https://doi.org/10.1210/er.2006-0037
Hanna RA, Quinsay MN, Orogo AM et al (2012) Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem 287(23):19094–19104. https://doi.org/10.1074/jbc.M111.322933
He C, Bassik MC, Moresi V et al (2012) Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481:511–515. https://doi.org/10.1038/nature10758
Iqbal S, Ostojic O, Singh K et al (2013) Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 48(6):963–970. https://doi.org/10.1002/mus.23838
Kang C, Yeo D, Ji LL (2016) Muscle immobilization activates mitophagy and disrupts mitochondrial dynamics in mice. Acta Physiol (oxf) 218(3):188–197. https://doi.org/10.1111/apha.12690
Kim I, Rodriguez-Enriquez S, Lemasters JJ (2007) Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys 462(2):245–253. https://doi.org/10.1016/j.abb.2007.03.034
Kirkwood SP, Munn EA, Brooks GA (1986) Mitochondrial reticulum in limb skeletal muscle. Am J Physiol 251:C395-402. https://doi.org/10.1152/ajpcell.1986.251.3.C395
Kirkwood SP, Packer L, Brooks GA (1987) Effects of endurance training on a mitochondrial reticulum in limb skeletal muscle. Arch Biochem Biophys 255(1):80–88. https://doi.org/10.1016/0003-9861(87)90296-7
Kissova I, Deffieu M, Manon S, Camougrand N (2004) Uth1p is involved in the autophagic degradation of mitochondria. J Biol Chem 279(37):39068–39074. https://doi.org/10.1074/jbc.M406960200
Koshiba T, Detmer SA, Kaiser JT et al (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305(5685):858–862. https://doi.org/10.1126/science.1099793
Krawiec BJ, Frost RA, Vary TC et al (2005) Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Metab 289(6):E969–E980
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Mammucari C, Milan G, Romanello V et al (2007) FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6(6):458–471. https://doi.org/10.1016/j.cmet.2007.11.001
Meeusen S, DeVay R, Block J et al (2006) Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell 127(2):383–395. https://doi.org/10.1016/j.cell.2006.09.021
Memme JM, Erlich AT, Phukan G, Hood DA (2021) Exercise and mitochondrial health. J Physiol 599(3):803–817. https://doi.org/10.1113/JP278853
Merry TL, Ristow M (2015) Mitohormesis in exercise training. Free Radic Biol Med 98:123–130. https://doi.org/10.1016/j.freeradbiomed.2015.11.032
Miledi R, Slater CR (1968) Some mitochondrial changes in denervated muscle. J Cell Sci 3(1):49–54. https://doi.org/10.1242/jcs.3.1.49
Miotto PM, McGlory C, Bahniwal R et al (2019) Supplementation with dietary ω-3 mitigates immobilization-induced reductions in skeletal muscle mitochondrial respiration in young women. FASEB J 33(7):8232–8240. https://doi.org/10.1096/fj.201900095R
Mitchell CJ, McGregor RA, D’Souza RF et al (2015) Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients 7(10):8685–8699. https://doi.org/10.3390/nu7105420
Mitchell CJ, D’Souza RF, Mitchell SM et al (2018) The impact of dairy protein during limb immobilization and recovery on muscle size and protein synthesis; a randomized controlled trial. J Appl Physiol (1985) 124(3):717–728. https://doi.org/10.1152/japplphysiol.00803.2017
Morita M, Prudent J, Basu K et al (2017) mTOR controls mitochondrial dynamics and cell survival via MTFP1. Mol Cell 67(6):922–935. https://doi.org/10.1016/j.molcel.2017.08.013
Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 10(7):458–467. https://doi.org/10.1038/nrm2708
Norrbom J, Sundberg CJ, Ameln H et al (2004) PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol (1985) 96(1):189–194. https://doi.org/10.1152/japplphysiol.00765.2003
Novak I, Kirkin V, McEwan DG et al (2010) Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep 11(1):45–51. https://doi.org/10.1038/embor.2009.256
Ono T, Isobe K, Nakada K, Hayashi J-I (2001) Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet 28(3):272–275. https://doi.org/10.1038/90116
Perry CGR, Lally J, Holloway GP et al (2010) Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588:4795–4810. https://doi.org/10.1113/jphysiol.2010.199448
Pich S, Bach D, Briones P et al (2005) The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet 14(11):1405–1415. https://doi.org/10.1093/hmg/ddi149
Pilegaard H, Saltin B, Neufer PD (2003) Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol 546:851–858. https://doi.org/10.1113/jphysiol.2002.034850
Pileggi CA, Hedges CP, D’Souza RF et al (2018) Exercise recovery increases skeletal muscle H2O2 emission and mitochondrial respiratory capacity following two-weeks of limb immobilization. Free Radic Biol Med 124:241–248. https://doi.org/10.1016/j.freeradbiomed.2018.06.012
Puigserver P, Wu Z, Park CW et al (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92(6):829–839. https://doi.org/10.1016/s0092-8674(00)81410-5
Romanello V, Sandri M (2013) Mitochondrial biogenesis and fragmentation as regulators of protein degradation in striated muscles. J Mol Cell Cardiol 55:64–72. https://doi.org/10.1016/j.yjmcc.2012.08.001
Salvadego D, Keramidas ME, Brocca L et al (2016) Separate and combined effects of a 10-d exposure to hypoxia and inactivity on oxidative function in vivo and mitochondrial respiration ex vivo in humans. J Appl Physiol (1985) 121(1):154–163. https://doi.org/10.1152/japplphysiol.00832.2015
Sarraf SA, Raman M, Guarani-Pereira V et al (2013) Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496(7445):372–376. https://doi.org/10.1038/nature12043
Schreiber SN, Emter R, Hock MB et al (2004) The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci USA 101:6472–6477. https://doi.org/10.1073/PNAS.0308686101
Shutt T, Geoffrion M, Milne R, McBride HM (2012) The intracellular redox state is a core determinant of mitochondrial fusion. EMBO Rep 13(1):909–915. https://doi.org/10.1038/embor.2012.128
Smith GA, Lin T-H, Sheehan AE et al (2019) Glutathione S-transferase regulates mitochondrial populations in axons through increased glutathione oxidation. Neuron 103(1):52–65. https://doi.org/10.1016/j.neuron.2019.04.017
Talbert EE, Smuder AJ, Min K et al (2013) Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant. J Appl Physiol (1985) 115(4):529–538. https://doi.org/10.1152/japplphysiol.00471.2013
Tondera D, Santel A, Schwarzer R et al (2004) Knockdown of MTP18, a novel phosphatidylinositol 3-kinase-dependent protein, affects mitochondrial morphology and induces apoptosis. J Biol Chem 279(30):31544–31555. https://doi.org/10.1074/jbc.M404704200
Tryon LD, Vainshtein A, Memme JM et al (2014) Recent advances in mitochondrial turnover during chronic muscle disuse. Integr Med Res 3(4):161–171. https://doi.org/10.1016/j.imr.2014.09.001
Twig G, Hyde B, Shirihai OS (2008) Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochim Biophys Acta Bioenerg 1777(9):1092–1097. https://doi.org/10.1016/j.bbabio.2008.05.001
Vazeille E, Codran A, Claustre A et al (2008) The ubiquitin-proteasome and the mitochondria-associated apoptotic pathways are sequentially downregulated during recovery after immobilization-induced muscle atrophy. Am J Physiol Endocrinol Metab 295(5):E1181–E1190. https://doi.org/10.1152/ajpendo.90532.2008
Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA 91(4):1309–1313. https://doi.org/10.1073/pnas.91.4.1309
Vives-Bauza C, Zhou C, Huang Y et al (2010) PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci USA 107(1):378–383. https://doi.org/10.1073/pnas.0911187107
Wright DC, Han D-H, Garcia-Roves PM et al (2007) Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem 282(1):194–199. https://doi.org/10.1074/jbc.M606116200
Wu Z, Puigserver P, Andersson U et al (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98(1):115–124. https://doi.org/10.1016/S0092-8674(00)80611-X
Yang J, Liu X, Bhalla K et al (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275(5303):1129–1132. https://doi.org/10.1126/science.275.5303.1129
Yang Y, Ouyang Y, Yang L et al (2008) Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci USA 105(19):7070–7075. https://doi.org/10.1073/pnas.0711845105
Ydfors M, Fischer H, Mascher H et al (2013) The truncated splice variants, NT-PGC-1alpha and PGC-1alpha4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol Rep 1(6):e00140. https://doi.org/10.1002/phy2.140
Youle RJ, Narendra DP (2011) Mechanisms of mitophagy. Nat Rev Mol Cell Biol 12(1):9–14. https://doi.org/10.1038/nrm3028
Zeng N, D’Souza RF, MacRae CL et al (2021) Daily protein supplementation attenuates immobilization-induced blunting of postabsorptive muscle mTORC1 activation in middle-aged men. Am J Physiol Physiol 320:C591–C601. https://doi.org/10.1152/ajpcell.00284.2020
Acknowledgements
The authors acknowledge the volunteers who so willingly contributed to this clinical experimental study. We also thank Aaron Fanning (Fonterra) and Professor Sally Poppitt for their contribution to the New Zealand Primary Growth Partnership (PGP) post-farm gate research program.
Funding
The overall project was funded by the New Zealand Ministry for Primary Industries (MPI) and Fonterra Co-operative Group Ltd through the Primary Growth Partnership (PGP) post-farm gate program, with additional analytical funding from AgResearch Limited through the Strategic Science Investment Fund (Contract Nos. A19079 and A21246: Nutritional strategies for an ageing population). CAP was supported by Ph.D. scholarships from AgResearch Limited and the Agnes Paykel trust.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: CAP, CJM, and DC-S; performed the experiments: CAP, NZ, BRD, RFD, VCF, and CJM; analysed data: CAP and CJM; drafted the manuscript: CAP; wrote the paper: CAP. Critically evaluated the paper: CAP, CPH, AJR, VCF, CJM, and DC-S. All authors edited and revised manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Additional information
Communicated by Håkan Westerblad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pileggi, C.A., Hedges, C.P., D’Souza, R.F. et al. Minimal adaptation of the molecular regulators of mitochondrial dynamics in response to unilateral limb immobilisation and retraining in middle-aged men. Eur J Appl Physiol 123, 249–260 (2023). https://doi.org/10.1007/s00421-022-05107-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-05107-x