Abstract
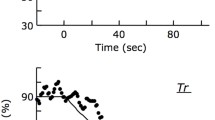
This study addressed whether O2 delivery during recovery from high-intensity, supra-gas exchange threshold exercise would be matched to O2 utilization at the microvascular level in patients with mitochondrial myopathy (MM). Off-exercise kinetics of (1) pulmonary O2 uptake \( (\dot{V}{\text{O}}_{2} {\text{p}}), \) (2) an index of fractional O2 extraction by near-infrared spectroscopy (Δ[deoxy-Hb + Mb]) in the vastus lateralis and (3) cardiac output (Q ′T ) by impedance cardiography were assessed in 12 patients with biopsy-proven MM (chronic progressive external ophthalmoplegia) and 12 age- and gender-matched controls. Kinetics of \( \dot{V}{\text{O}}_{2} {\text{p}} \) were significantly slower in patients than controls (τ = 53.8 ± 16.5 vs. 38.8 ± 7.6 s, respectively; p < 0.05). Q ′T , however, declined at similar rates (τ = 64.7 ± 18.8 vs. 73.0 ± 21.6 s; p > 0.05) being typically slower than \( \dot{V}{\text{O}}_{2} {\text{p}} \) in both groups. Importantly, Δ[deoxy-Hb + Mb] dynamics (MRT) were equal to, or faster than, \( \tau \dot{V}{\text{O}}_{2} {\text{p}} \) in patients and controls, respectively. In fact, there were no between-group differences in \( \tau \dot{V}{\text{O}}_{2} {\text{p}} \)/MRTΔ[deoxy-Hb + Mb] (1.1 ± 0.4 vs. 1.0 ± 0.2, p > 0.05) thereby indicating similar rates of microvascular O2 delivery. These data indicate that the slower rate of recovery of muscle metabolism after high-intensity exercise is not related to impaired microvascular O2 delivery in patients with MM. This phenomenon, therefore, seems to reflect the intra-myocyte abnormalities that characterize this patient population.


Similar content being viewed by others
References
Baecke JA, Burema J, Frijters JER (1982) A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36(5):936–942
Bank W, Chance B (1997) Diagnosis of defects in oxidative muscle metabolism by non-invasive tissue oximetry. Mol Cell Biochem 174:7–10
Beaver WL, Wasserman K, Whipp BJA (1986) New method for detecting the anaerobic threshold by gas exchange. J Appl Physiol 60:2020–2027
Behnke BJ, Ferreira LF, McDonough PJ, Musch TI, Poole DC (2009) Recovery dynamics of skeletal muscle oxygen uptake during the exercise off-transient. Respir Physiol Neurobiol 168(3):254–260
Borghi-Silva A, Oliveira CC, Carrascosa C, Maia J, Berton DC, Queiroga F Jr, Ferreira EM, Almeida DR, Nery LE, Neder JA (2008) Respiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPD. Thorax 63(10):910–915
Burnley M, Jones AM, Carter H, Doust JH (2000) Effects of prior heavy exercise on phase II pulmonary oxygen uptake kinetics during heavy exercise. J Appl Physiol 89:1387–1396
Charloux A, Lonsdorfer-Wolf E, Richard R, Lampert E, Oswald-Mammosser M, Mettauer B, Geny B, Lonsdorfer J (2000) A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” Fick method. Eur J Appl Physiol 82:313–320
Cleuziou C, Perrey S, Borrani F, Lecoq AM, Candau R, Courteix D, Obert P (2004) Dynamic responses of O2 uptake at the onset and end of exercise in trained subjects. Can J Appl Physiol 28(4):630–641
DeLorey DS, Kowalchuk JM, Paterson DH (2003) Relationship between pulmonary O2 uptake kinetics and muscle deoxygenation during moderate-intensity exercise. J Appl Physiol 95(1):113–120
DeLorey DS, Kowalchuk JM, Paterson DH (2005) Adaptation of pulmonary O2 uptake kinetics and muscle deoxygenation at the onset of heavy-intensity exercise in young and older adults. J Appl Physiol 98:1697–1704
Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS (2009) Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology 24:107–116
Ferrari M, Mottola L, Quaresima V (2004) Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol 29:463–487
Ferreira LF, Harper AJ, Townsend DK, Lutjemeier BJ, Barstow TJ (2005) Kinetics of estimated human muscle capillary blood flow during recovery from exercise. Exp Physiol 90(5):715–726
Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD (1996) Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol 80:988–998
Grassi B, Pogliaghi S, Rampichini S, Quaresima V, Ferrari M, Marconi C, Cerretelli P (2003) Muscle oxygenation and pulmonary gas exchange kinetics during cycling exercise on-transitions in humans. J Appl Physiol 95:149–158
Grassi B, Marzorati M, Lanfranconi F, Ferri A, Longaretti M, Stucchi A, Vago P, Marconi C, Morandi L (2007) Impaired oxygen extraction in metabolic myopathies: detection and quantification by near-infrared spectroscopy. Muscle Nerve 35:510–520
Grassi B, Porcelli S, Marzorati M, Lanfranconi F, Vago P, Marconi C, Morandi L (2009) Metabolic myopathies: functional evaluation by analysis of oxygen uptake kinetics. Med Sci Sports Exerc 41(12):2120–2127
Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ (2008) Matching of blood flow to metabolic rate during recovery from moderate exercise in humans. Exp Physiol 93(10):1118–1125
Harris RC, Edwards RHT, Hultman E, Nordesjo LO, Nylind B, Sahlin K (1976) The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pfluegers Arch 367:137–142
Hughson RL, Tschakovsky ME, Houston ME (2001) Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev 29:129–133
Jensen TD, Kazemi-Esfarjani P, Skomorowska E, Vissing J (2002) A forearm exercise screening test for mitochondrial myopathy. Neurology 58:1533–1538
Jeppesen TD, Quistorff B, Wibrand F, Vissing J (2007) 31P-MRS of skeletal muscle is not a sensitive diagnostic test for mitochondrial myopathy. J Neurol 254(1):29–37
Kemp GJ, Taylor DJ, Thompson CH, Hauds LJ, Rajagopalan B, Styles P, Radda GK (1993) Quantitative analysis by 31P magnetic resonance spectroscopy of abnormal mitochondrial oxidation in skeletal muscle during recovery from exercise. NMR Biomed 6(5):302–310
Kemps HM, Thijssen EJ, Schep G, Sleutjes BT, De Vries WR, Hoogeveen AR, Wijn PF, Doevendans PA (2008) Evaluation of two methods for continuous cardiac output assessment during exercise in chronic heart failure patients. J Appl Physiol 105(6):1822–1829
Kemps HM, Prompers JJ, Wessels B, De Vries WR, Zonderland ML, Thijssen EJ, Nicolay K, Schep G, Doevendans PA (2009) Skeletal muscle metabolic recovery following submaximal exercise in chronic heart failure is limited more by O2 delivery than O2 utilization. Clin Sci 26 118(3):203–210
Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ (2007) Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103:2049–2056
MacDonald MJ, Naylor HL, Tschakovsky ME, Hughson RL (2001) Peripheral circulatory factors limit rate of increase in muscle O2 uptake at onset of heavy exercise. J Appl Physiol 90:83–89
Matsushita K, Homma S, Okada E (1998) Influence of adipose tissue on muscle oxygenation measurement with NIRS instrument. Proc SPIE 3194:151–165
McDonough P, Behnke BJ, Musch TI, Poole DC (2004) Recovery of microvascular PO2 during the exercise off-transient in muscles of different fiber type. J Appl Physiol 96:1039–1044
McMahon S, Jenkins D (2002) Factors affecting the rate of phosphocreatine resynthesis following intense exercise. Sports Med 32(12):761–784
Moraes CT, DiMauro S, Zeviani M, Lombes A, Shanske S, Miranda AF, Nakase H, Bonilla E, Werneck LC, Servidei S, Nonaka I, Koga Y, Spiro AJ, Brownell AKW, Schmidt B, Schotland DL, Zupanc M, DeVivo DC, Schon EA, Rowland LP (1989) Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns–Sayre syndrome. N Engl J Med 320:1293–1299
Ozyener F, Rossiter HB, Ward SA, Whipp BJ (2001) Influence of exercise intensity on the on- and off-transient kinetics of pulmonary oxygen uptake in humans. J Physiol 533(3):891–902
Perrey S, Candau R, Borrani F, Millet GY, Rouillon JD (2002) Recovery kinetics of oxygen uptake following severe-intensity exercise in runners. J Sports Med Phys Fit 42(4):381–388
Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ (2002) Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate and high-intensity exercise in humans. J Physiol 541(3):991–1002
Sakuta R, Nonaka I (1989) Vascular involvement in mitochondrial myopathy. Ann Neurol 25(6):594–601
Scelsi R (1992) Morphometric analysis of skeletal muscle fibres and capillaries in mitochondrial myopathies. Path Res Pract 188:607–611
Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML (2009) Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep 61:183–190
Sprague RS, Bowles EA, Achilleus D, Ellsworth ML (2010) Erythrocytes as controllers of perfusion distribution in the microvasculature of skeletal muscle. Acta Physiol 22. doi: 10.1111/j.1748-1716.2010.02182.x. (Epub ahead of print)
Taivassalo T, Abbott A, Wyrick P, Haller RG (2002) Venous oxygen levels during aerobic forearm exercise: an index of impaired oxidative metabolism in mitochondrial myopathy. Ann Neurol 51:38–44
Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG (2003) The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain 126:413–423
Tarnopolski M (2004) Exercise testing as a diagnostic entity in mitochondrial myopathies. Mitochondrion 4:529–542
Tarnopolski M, Raha S (2005) Mitochondrial myopathies: diagnosis, exercise intolerance, and treatment options. Med Sci Sports Exerc 37:2086–2093
Tonelli AR, Alnuaimat H, Li N, Carrie R, Mubarak KK (2011) Value of Impedance Cardiography in Patients Studied for Pulmonary Hypertension. Lung. doi:10.1007/s00408-011-9299-y
Trenell MI, Sue CM, Thompson CH, Kemp GJ (2007) Supplemental oxygen and muscle metabolism in mitochondrial myopathy patients. Eur J Appl Physiol 99:541–547
Van Beekvelt MC, Colier WN, Wevers RA, Van Engelen BG (1999a) Quantitative measurement of oxygen consumption and forearm blood flow in patients with mitochondrial myopathies. Adv Exp Med Biol 471:313–319
Van Beekvelt MC, Van Engelen BG, Wevers RA, Colier WN (1999b) Quantitative near-infrared spectroscopy discriminates between mitochondrial myopathies and normal muscle. Ann Neurol 46(4):667–670
Van Beekvelt MC, Van Engelen BG, Wevers RA, Colier WN (2002) Near-infrared spectroscopy in chronic progressive external ophthalmoplegia: adipose tissue thickness confounds decreased muscle oxygen consumption. Ann Neurol 51(2):272–273
Whipp BJ, Davis JA, Torres F, Wasserman K (1981) A test to determine parameters of aerobic function during exercise. J Appl Physiol 50:217–221
Wolin MS (2009) Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296(3):H539–H549
Acknowledgments
The authors would like to thank all colleagues from the Pulmonary Function and Clinical Exercise Physiology Unit [Division of Respiratory Diseases. Department of Medicine, Federal University of Sao Paulo (UNIFESP), Brazil] for their friendly collaboration and support obtained from FAPESP (Fundação de Amparo à Pesquisa do Estado de Sao Paulo, Sao Paulo, Brazil) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil). Supported by a Research Grant from FAPESP (Fundação de Amparo à Pesquisa do Estado de Sao Paulo, Sao Paulo, Brazil). DMB was a recipient of a Master Scholarship Grant from FAPESP. JAN and LEN are Established Investigators (level II) of the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Susan A. Ward.
Rights and permissions
About this article
Cite this article
Bravo, D.M., Gimenes, A.C., Nascimento, R.B. et al. Skeletal muscle reoxygenation after high-intensity exercise in mitochondrial myopathy. Eur J Appl Physiol 112, 1763–1771 (2012). https://doi.org/10.1007/s00421-011-2136-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-011-2136-4