Abstract
Cold-induced vasodilation (CIVD) is an acute increase in peripheral blood flow observed during cold exposures. It is hypothesized to protect against cold injuries, yet despite continuous research it remains an unexplained phenomenon. Contrary to the traditionally held view, we propose that CIVD is a thermoregulatory reflex mechanism contributing to heat loss. Ten adults (4 females; 23.8 ± 2.0 years) randomly underwent three 130-min exposures to −20°C incorporating a 10-min moderate exercise period at the 65th min, while wearing a liquid conditioning garment (LCG) and military arctic clothing. In the pre-warming condition, rectal temperature was increased by 0.5°C via the LCG before the cold exposure. In the warming condition, participants regulated the LCG throughout the cold exposure to subjective comfort. In the control condition, the LCG was worn but was not operated either before or during the cold exposure. Results demonstrated that the majority of CIVD occurred during the warming condition when the thermometrically-estimated mean body temperature (T b) was at its highest. A thermoregulatory pattern was identified whereby CIVD occurred soon after T b increased past a threshold (~36.65°C in warming and pre-warming; ~36.4°C in control). When CIVD occurred, T b was reduced and CIVD ceased when T b fell below the threshold. These findings were independent of extremity temperature since CIVD episodes occurred at a large range of finger temperatures (7.2–33.5°C). These observations were statistically confirmed by auto-regressive integrated moving average analysis (t = 9.602, P < 0.001). We conclude that CIVD is triggered by increased T b supporting the hypothesis that CIVD is a thermoregulatory mechanism contributing to heat loss.




Similar content being viewed by others
References
Box GEP, Jenkins GM (1976) Time series analysis: forecasting and control, rev. ed. Holden-Day, San Francisco
Cannon P, Keatinge WR (1960) The metabolic rate and heat loss of fat and thin men in heat balance in cold and warm water. J Physiol 154:329–344
Caputa M, Cabanac M (1980) Muscular work as thermal behavior in humans. J Appl Physiol 48:1020–1023
Charkoudian N (2003) Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 78:603–612
Cheung SS, Mekjavic IB (2007) Cold-induced vasodilatation is not homogenous or generalizable across the hand and feet. Eur J Appl Physiol 99:701–705
Daanen HA (2003) Finger cold-induced vasodilation: a review. Eur J Appl Physiol 89:411–426
Daanen HA, Ducharme MB (1999) Finger cold-induced vasodilation during mild hypothermia, hyperthermia and at thermoneutrality. Aviat Space Environ Med 70:1206–1210
Daanen HA, Van de Linde FJ, Romet TT, Ducharme MB (1997) The effect of body temperature on the hunting response of the middle finger skin temperature. Eur J Appl Physiol Occup Physiol 76:538–543
Daanen HA, van der Struijs NR (2005) Resistance index of frostbite as a predictor of cold injury in arctic operations. Aviat Space Environ Med 76:1119–1122
Elsner RW, Nelms JD, Irving L (1960) Circulation of heart to the hands of Arctic Indians. J Appl Physiol 15:662–666
Eyolfson DA, Tikuisis P, Xu X, Weseen G, Giesbrecht GG (2001) Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol 84:100–106
Flouris AD, Cheung SS (2006) Design and control optimization of microclimate liquid cooling systems underneath protective clothing. Ann Biomed Eng 34:359–372
Flouris AD, Westwood DA, Cheung SS (2007) Thermal balance effects on vigilance during 2-hour exposures to −20°C. Aviat Space Environ Med 78:673–679
Gardner ES (1985) Exponential smoothing: the state of the art. J Forecast 4:1–28
Grant RT, Bland E (1931) Observations on arterio-venous anastomoses in human skin and in the bird’s foot with special reference to the reaction to cold. Heart 15:385–411
Haman F, Peronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, Weber JM (2002) Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol 93:77–84
Hardy JD, DuBois EF (1938) Basal metabolism, radiation, convection, and vapourization at temperatures 22°C to 35°C. J Nutr 15:477
Hellstrom B, Andersen KL (1960) Heat output in the cold from hands of Arctic fishermen. J Appl Physiol 15:771–775
Johnson JM, Park MK (1979) Reflex control of skin blood flow by skin temperature: role of core temperature. J Appl Physiol 47:1188–1193
Johnson JM, Yen TC, Zhao K, Kosiba WA (2005) Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 288:H1573–H1579
Keatinge WR (1957) The effect of general chilling on the vasodilator response to cold. J Physiol 139:497–507
Keatinge WR (1980) Effects of temperature on blood vessels. In: Local mechanisms controlling blood vessels. Cambridge University Press, London
Kellogg DL Jr (2006) In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol 100:1709–1718
Krog J, Folkow B, Fox RH, Andersen KL (1960) Hand circulation in the cold of Lapps and North Norwegian fisherman. J Appl Physiol 15:654–658
Kunimoto M (1987) Where is the most useful point in the skin temperature curve of the fingertip immersed in the cold water to evaluate the function of the skin sympathetic function? Biomed Thermogr 7:254–268
Leblanc J, Hildes JA, Heroux O (1960) Tolerance of Gaspe fishermen to cold water. J Appl Physiol 15:1031–1034
Lossius K, Eriksen M, Walloe L (1994) Thermoregulatory fluctuations in heart rate and blood pressure in humans: effect of cooling and parasympathetic blockade. J Auton Nerv Syst 47:245–254
Lossius K, Eriksen M, Walløe L (1993) Fluctuations in blood flow to acral skin in humans: connection with heart rate and blood pressure variability. J Physiol 460:641–655
Michikami D, Iwase S, Kamiya A, Qi F, Mano T, Suzumura A (2001) Interrelations of vasoconstrictor sympathetic outflow to skin core temperature during unilateral sole heating in humans. Auton Neurosci 91:55–61
Nelms JD, Soper DJ (1962) Cold vasodilatation and cold acclimatization in the hands of British fish filleters. J Appl Physiol 17:444–448
Olsen J (2003) What characterises a useful concept of causation in epidemiology? J Epidemiol Community Health 57:86–88
Purkayastha SS, Selvamurthy W, Ilavazhagan G (1992) Peripheral vascular response to local cold stress of tropical men during sojourn in the Arctic cold region. Jpn J Physiol 42:877–889
Ramanathan NL (1964) A new weighting system for mean surface temperature of the human body. J Appl Physiol 19:531–533
Reynolds LF, Mekjavic IB, Cheung SS (2007) Cold-induced vasodilatation in the foot is not homogenous or trainable over repeated cold exposure. Eur J Appl Physiol 102(1):73–78
Roberts MF, Zygmunt AC (1984) Reflex and local thermal control of rabbit ear blood flow. Am J Physiol 246:R979–R984
Schwinghamer JM, Adams T (1969) Cold-induced vasodilation in the footpad of the anesthetized cat. Fed Proc 28:1149–1154
Sendowski I, Savourey G, Besnard Y, Bittel J (1997) Cold induced vasodilatation and cardiovascular responses in humans during cold water immersion of various upper limb areas. Eur J Appl Physiol Occup Physiol 75:471–477
Sendowski I, Savourey G, Launay JC, Besnard Y, Cottet-Emard JM, Pequignot JM, Bittel J (2000) Sympathetic stimulation induced by hand cooling alters cold-induced vasodilatation in humans. Eur J Appl Physiol 81:303–309
Sessler DI (2003) Skin-temperature gradients are a validated measure of fingertip perfusion. Eur J Appl Physiol 89:401–402 author’s reply 403–404
Takano N, Kotani M (1989) Influence of food intake on cold-induced vasodilatation of finger. Jpn J Physiol 39:755–765
Thompson-Torgerson CS, Holowatz LA, Flavahan NA, Kenney WL (2007) Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol 292:H1700–H1705
Tipton MJ, Allsopp A, Balmi PJ, House JR (1993) Hand immersion as a method of cooling and rewarming: a short review. J R Nav Med Serv 79:125–131
Werner J (1977) Influences of local and global temperature stimuli on the Lewis-reaction. Pflugers Arch 367:291–294
Wilson LM, Currie PJ, Gilson TL (1991) Thermal preference behavior in preweaning genetically obese (ob/ob) and lean (+/?, +/+) mice. Physiol Behav 50:155–160
Acknowledgments
The authors wish to express their gratitude to the subjects who participated in the experiments, to Peter Romkey and the K.C. Irving Center at Acadia University for providing the environmental chamber and associated technical assistance, and to Dr. René J.L. Murphy at Acadia University for providing the arctic clothing. The project was supported by a Discovery Grant (S. S. Cheung and G. G. Sleivert) from the Natural Sciences and Engineering Research Council (NSERC). A. D. Flouris was supported by funding from the Natural Sciences and Engineering Research Council of Canada and the Canadian Space Agency.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
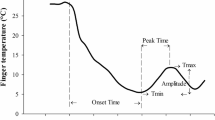
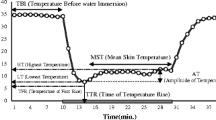
Plotting the T f time series against the T b time series for each participant and condition separately (Figs. 2–4) yielded a systematic pattern (relationship between T f and T b described above) but no periodicity [i.e., regular repetition in time (lack of periodicity is an essential ARIMA assumption)] (Box and Jenkins 1976). Further analyses were conducted using data from all participants and conditions simultaneously. To statistically address the possibility that the two time series (i.e., T f and T b) were not inherently unpredictable [random-walk/white noise (another ARIMA assumption)], the autocorrelation of the residuals from exponential smoothing (Gardner 1985) was calculated for each time series for a maximum of 38 lags/data points (i.e., ~5 min, given that data were collected every 8 s), confirming that neither of the two was random (Box-Ljung statistic P < 0.001). The exponential smoothing technique is very useful in time series analysis as it emphasizes the regularity of a series by removing some of the random variation and by giving extra weight to more recent observations. This allows the researcher to capitalize on any pattern that is evident in the observed series and to use that pattern to draw relevant conclusions. In the current analysis, the changes in T f were detected approximately 160 s (or 20 lags/data points) following changes in T b. Thus, the T f series was recalculated to incorporate 20 lags and further analyses were conducted using this new variable.
ARIMA models combine as many as three types of processes based on the concept of random disturbances or shocks: autoregression (i.e., AR), integration/differencing (i.e., I) and moving averages (i.e., MA). Between two observations in a time series, a disturbance (in this case cold exposure/LCG heating) occurs that somehow affects the level of the series (in this case T b) as well as the level of another series (in this case T f). These disturbances as well as the association between the two time series can be mathematically described by ARIMA models. Using the appropriate model-building procedure for the best possible series model (Box and Jenkins 1976) the identification of the processes underlying the time series was conducted to determine the integers for autoregression, differencing and moving average. Given that the autoregression and moving average components require stationary series (Box and Jenkins 1976), the autocorrelations and partial autocorrelations of T f and T b time series were calculated showing that neither was stationary. This was also evident in all preliminary plots of T f and T b across time (Figs. 2–4). Following differencing transformation at lag 2, stationarity was achieved with no spikes evident to the 38th lag (~5 min of data collection given that data were collected every 8 s). As the series were differenced twice to achieve stationarity, the ARIMA integration parameter was set to ‘2’ (Box and Jenkins 1976). Further, given that both series had one spike in the first value of the autocorrelation and exponentially declining values of the partial autocorrelation, the appropriate order for autoregression and moving average was ‘0’ and ‘1’, respectively (Box and Jenkins 1976). The resulting ARIMA [0, 2, 1 (i.e., 0 order of autoregression; 2 degree of differencing; 1 order of moving average)] was found to be parsimonious and adequate model with no spikes evident to the 38th lag (Box-Ljung statistic P < 0.001) and T b significantly associated with T f (t = 9.602, P < 0.001). These results demonstrate that, across time, fluctuations in T b were systematically followed (approximately 160 s later) by similar fluctuations in T f.
Rights and permissions
About this article
Cite this article
Flouris, A.D., Westwood, D.A., Mekjavic, I.B. et al. Effect of body temperature on cold induced vasodilation. Eur J Appl Physiol 104, 491–499 (2008). https://doi.org/10.1007/s00421-008-0798-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-008-0798-3