Abstract
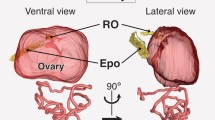
The male genital tract is diverse among vertebrates, but its development remains unclear, especially in the rete region. In this study, we investigated the testis–mesonephros complex of rabbit, chicken, and frog (Xenopus tropicalis) by immunohistochemistry for markers such as Ad4BP/Sf-1 (gonadal somatic and rete cells in mammals) and Pax2 (mesonephric tubules), and performed a three-dimensional reconstruction. In all investigated animals, testis cords were bundled at the mesonephros side. Rete cells positive for Ad4BP/Sf-1 (rabbit) or Pax2 (chicken and frog) were clustered at the border region between the testis and mesonephros. The cluster possessed two types of cords; one connected to the testis cords and the other to the mesonephric tubules. The latter rete cords were contiguous to Bowman’s capsules in rabbit and chicken but to nephrostomes in frog. In conclusion, this study showed that mammals, avian species, and frogs commonly develop the bundle between the testis cords (testis canal) and the cluster of rete cells (lateral kidney canal), indicating that these animals share basic morphogenesis in the male genital tract. The connection site between the rete cells and mesonephric tubules is suggested to have changed from the nephrostome to the Bowman’s capsule during vertebrate evolution from anamniote to amniote.







Similar content being viewed by others
Data availability
The materials in this study are commercially available, except for the goat anti-AMH antibody.
References
Budras KD, Meier U (1981) The epididymis and its development in ratite birds (ostrich, emu, rhea). Anat Embryol 162:281–299. https://doi.org/10.1007/BF00299973
Budras K-D, Sauer T (1975) Morphology of the epididymis of the cock (Gallus domesticus) and its effect upon the steroid sex hormone synthesis. Anat Embryol 148:175–196. https://doi.org/10.1007/BF00315268
Cao Y, Liu L, Lin J et al (2021) Dysregulation of Notch-FGF signaling axis in germ cells results in cystic dilation of the rete testis in mice. J Cell Commun Signal. https://doi.org/10.1007/s12079-021-00628-0
Estermann MA, Williams S, Hirst CE et al (2020) Insights into gonadal sex differentiation provided by single-cell transcriptomics in the chicken embryo. Cell Rep 31:107491. https://doi.org/10.1016/j.celrep.2020.03.055
Gray P (1936) Memoirs: the development of the amphibian kidney. Part III. The post-metamorphic development of the kidney, and the development of the vasa efferentia and seminal vesicles in rana temporaria. J Cell Sci. https://doi.org/10.1242/jcs.s2-78.311.445
Hahn KL, Beres B, Rowton MJ et al (2009) A deficiency of lunatic fringe is associated with cystic dilation of the rete testis. J Reprod Fertil 137:79–93. https://doi.org/10.1530/rep-08-0207
Harikae K, Miura K, Kanai Y (2013) Early gonadogenesis in mammals: significance of long and narrow gonadal structure. Dev Dyn 242:330–338. https://doi.org/10.1002/dvdy.23872
Hiragond NC, Saidapur SK (2000) The excurrent duct system of sperm transport in Rana cyanophlyctis, Rana limnocharis, Polypedates maculatus, Microhyla rubra, Bufo melanostictus and Bufo fergusonii. Zool Sci 17:453–458. https://doi.org/10.2108/0289-0003(2000)17[453:TEDSOS]2.0.CO;2
Ilio KY, Hess RA (1994) Structure and function of the ductuli efferentes: a review. Microsc Res Tech 29:432–467. https://doi.org/10.1002/jemt.1070290604
Imura-Kishi K, Uchida A, Tsunekawa N et al (2021) Low retinoic acid levels mediate regionalization of the Sertoli valve in the terminal segment of mouse seminiferous tubules. Sci Rep 11:1110. https://doi.org/10.1038/s41598-020-79987-4
Karl J, Capel B (1995) Three-dimensional structure of the developing mouse genital ridge. Philos Trans R Soc Lond B Biol Sci 350:235–242. https://doi.org/10.1098/rstb.1995.0157
Kulibin AY, Malolina EA (2020) Formation of the rete testis during mouse embryonic development. Dev Dyn 249:1486–1499. https://doi.org/10.1002/dvdy.242
Lake PE (1957) The male reproductive tract of the fowl. J Anat 91:116–129
Li Y, Li J, Cai M, Qin Z (2020) Development of testis cords and the formation of efferent ducts in Xenopus laevis: differences and similarities with other vertebrates. Sex Dev 14:66–79. https://doi.org/10.1159/000513416
Liem KF, Walker WF, Bemis WE, Grande L (2001) Functional Anatomy of the vertebrates: an evolutionary perspective, third. Harcourt College Publishers, Fort Worth
Luo X, Ikeda Y, Parker KL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490. https://doi.org/10.1016/0092-8674(94)90211-9
Major AT, Estermann MA, Smith CA (2021) Anatomy, endocrine regulation and embryonic development of the rete testis. Endocrinology. https://doi.org/10.1210/endocr/bqab046
Nakata H, Iseki S (2019) Three-dimensional structure of efferent and epididymal ducts in mice. J Anat 235:271–280. https://doi.org/10.1111/joa.13006
Oielska M (2009) Reproduction of amphibians. CRC Press, Boca Raton
Omotehara T, Minami K, Mantani Y et al (2017) Contribution of the coelomic epithelial cells specific to the left testis in the chicken embryo. Dev Dyn 246:148–156. https://doi.org/10.1002/dvdy.24469
Omotehara T, Wu X, Kuramasu M, Itoh M (2020) Connection between seminiferous tubules and epididymal duct is originally induced before sex differentiation in a sex-independent manner. Dev Dyn 249:754–764. https://doi.org/10.1002/dvdy.155
Omotehara T, Nakata H, Itoh M (2021) Three-dimensional analysis of mesonephric tubules remodeling into efferent tubules in the male mouse embryo. Dev Dyn. https://doi.org/10.1002/dvdy.410
Romer AS, Parsons TS (1977) The vertebrate body, fifth. W. B Saunders Company, Philadelphia
Rugh R (1951) The frog: its reproduction and development. The Blakiston Company, Toronto
Satoh M (1991) Histogenesis and organogenesis of the gonad in human embryos. J Anat 177:85–107
Sekido R, Lovell-Badge R (2007) Mechanisms of gonadal morphogenesis are not conserved between chick and mouse. Dev Biol 302:132–142. https://doi.org/10.1016/j.ydbio.2006.09.007
Siegel DS, Aldridge RD, Rheubert JL et al (2013) The testicular sperm ducts and genital kidney of male Ambystoma maculatum (Amphibia, Urodela, Ambystomatidae). J Morphol 274:344–360. https://doi.org/10.1002/jmor.20100
Tanimura A, Iwasawa H (1988) Ultrastructural observations on the origin and differentiation of somatic cells during gonadal development in the frog Rana nigromaculata. (frog/gonadal development/sex differentiation). Dev Growth Differ 30:681–691. https://doi.org/10.1111/j.1440-169x.1988.00681.x
Tanimura A, Iwasawa H (1989) Origin of somatic cells and histogenesis in the primordial gonad of the Japanese tree frog Rhacophorus arboreus. Anat Embryol 180:165–173. https://doi.org/10.1007/BF00309768
Wartenberg H, Kinsky I, Viebahn C, Schmolke C (1991) Fine structural characteristics of testicular cord formation in the developing rabbit gonad. J Electron Microsc Tech 19:133–157. https://doi.org/10.1002/jemt.1060190203
Wiedersheim R (1907) Comparative anatomy of vertebrates (trans. WN Parker) third. Macmillan & co. Ltd, London
Wrobel KH (2001) Morphogenesis of the bovine rete testis: extratesticular rete, mesonephros and establishment of the definitive urogenital junction. Anat Embryol 203:293–307. https://doi.org/10.1007/s004290100162
Zamboni L, Upadhyay S (1982) The contribution of the mesonephros to the development of the sheep fetal testis. Am J Anat 165:339–356. https://doi.org/10.1002/aja.1001650309
Acknowledgements
We sincerely appreciate Professor Koichiro Ichimura for his advice on the use of the rabbit mesonephros. Xenopus tropicalis was provided by Hiroshima University Amphibian Research Center through National BioResource Project (NBRP) of AMED under Grant Number JP20km0210085. We appreciate Mr. Shuichi Yamazaki for his technical assistance in making serial paraffin sections. We also thank Ms. Xi Wu, Ms. Miyuki Kuramasu, and Ms. Yuki Ogawa for their assistance in routine immunohistochemistry procedures, image processing, and secretary works. This study was supported by JSPS KAKENHI under Grant Number JP19K16483.
Funding
JSPS KAKENHI; Grant Number JP19K16483.
Author information
Authors and Affiliations
Contributions
T.O. conceived this study, performed experiments, evaluated the data, and wrote the manuscript. H.N. evaluated three-dimensional results and wrote and reviewed the manuscript. K.N. performed experiments and reviewed the manuscript. M.I. wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All animal experiments were performed according to protocols approved by the Tokyo Medical University Committee.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omotehara, T., Nakata, H., Nagahori, K. et al. Comparative anatomy on the development of sperm transporting pathway between the testis and mesonephros. Histochem Cell Biol 157, 321–332 (2022). https://doi.org/10.1007/s00418-021-02057-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-021-02057-x