Abstract
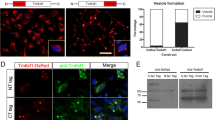
Lysosomal-associated membrane protein 1 (LAMP1) mainly localizes to lysosomes and late endosomes. We herein investigated the intracellular localization of lysosomal membrane proteins in five mammalian cultured cell lines. Rat LAMP1 fused to enhanced green fluorescent protein (EGFP) mostly accumulated at a particular cytoplasmic area and barely co-localized with LysoTracker® Red DND-99 in golden hamster kidney BHK-21 cells and Chinese hamster ovary CHO-K1 cells. Golden hamster, Chinese hamster, and human LAMP1-EGFP showed a similar intracellular distribution to rat LAMP1-EGFP in BHK-21 cells. Endogenous LAMP1 was also detected in a perinuclear area in BHK-21 cells and CHO-K1 cells, and co-localized with rat CD63-EGFP in BHK-21 cells. Moreover, rat LAMP1-DsRed-Monomer co-localized well with the human trans-Golgi network protein 2-EGFP in BHK-21 cells. These results reveal that LAMP1 predominantly localizes to the trans-Golgi network in BHK-21 cells.








Similar content being viewed by others
Change history
07 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00418-021-02010-y
References
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22
Aniento F, Emans N, Griffiths G, Gruenberg J (1993) Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol 123:1373–1387
Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72:395–447
Boonen M, Hamer I, Boussac M, Delsaute AF, Flamion B, Garin J, Jadot M (2006) Intracellular localization of p40, a protein identified in a preparation of lysosomal membranes. Biochem J 395:39–47
Chia PZC, Gleeson PA (2011) The regulation of endosome-to-Golgi retrograde transport by tethers and scaffolds. Traffic 12:939–947
Eskelinen EL (2006) Roles of LAMP-1 and LAMP-2 in lysosome biogenesis and autophagy. Mol Aspects Med 27:495–502
Eskelinen EL, Tanaka Y, Saftig P (2003) At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol 13:137–145
Eskelinen EL, Schmidt CK, Neu S, Willenborg M, Fuertes G, Salvador N, Tanaka Y, Lüllmann-Rauch R, Hartmann D, Heeren J, von Figura K, Knecht E, Saftig P (2004) Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol Biol Cell 15:3132–3145
Fukuda M (1991) Lysosomal membrane glycoproteins. J Biol Chem 266:21327–21330
Ghosh P, Dahms NM, Kornfeld S (2003) Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol 4:202–212
Gruenberg J, van der Goot FG (2006) Mechanisms of pathogen entry through the endosomal compartments. Nat Rev Mol Cell Biol 7:495–504
Hirst J, Edgar JR, Esteves T, Darios F, Madeo M, Chang J, Roda RH, Dürr A, Anheim M, Gellera C, Li J, Züchner S, Mariotti C, Stevanin G, Blackstone C, Kruer MC, Robinson MS (2015) Loss of AP-5 results in accumulation of aberrant endolysosomes: defining a new type of lysosomal storage disease. Hum Mol Genet 24:4984–4996
Humphrey JS, Peters PJ, Yuan LC, Bonifacino JS (1993) Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol 120:1123–1135
Janvier K, Bonifacino JS (2005) Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol Biol Cell 16:4231–4242
Jing J, Junutula JR, Wu C, Burden J, Matern H, Peden AA, Prekeris R (2010) FIP1/RCP binding to golgin-97 regulates retrograde transport from recycling endosomes to the trans-Golgi network. Mol Biol Cell 21:3041–3053
Kauppi M, Simonsen A, Bremnes B, Vieira A, Callaghan J, Stenmark H, Olkkonen VM (2002) The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. J Cell Sci 115:899–911
Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J (1998) A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392:193–197
Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J (1999) Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol 1:113–118
Kundra R, Kornfeld S (1999) Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem 274:31039–31046
Lieu ZZ, Derby MC, Teasdale RD, Hart C, Gunn P, Gleeson PA (2007) The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network. Mol Biol Cell 18:4979–4991
Lübke T, Lobel P, Sleat D (2009) Proteomics of the lysosome. Biochim Biophys Acta 1793:625–635
Luzio JP, Brake B, Banting G, Howell KE, Braghetta P, Stanley KK (1990) Identification, sequencing and expression of an integral membrane protein of the trans-Golgi network (TGN38). Biochem J 270:97–102
Luzio JP, Pryor PR, Bright NA (2007) Lysosomes: fusion and function. Nat Rev Mol Cell Biol 8:622–632
Matsubara H, Tanaka R, Tateishi T, Yoshida H, Yamaguchi M, Kataoka T (2019) The human Bcl-2 family member Bcl-rambo and voltage-dependent anion channels manifest a genetic interaction in Drosophila and cooperatively promote the activation of effector caspases in human cultured cells. Exp Cell Res 381:223–234
Matsuda I, Matsuo K, Matsushita Y, Haruna Y, Niwa M, Kataoka T (2014) The C-terminal domain of the long form of cellular FLICE-inhibitory protein (c-FLIPL) inhibits the interaction of the caspase 8 prodomain with the receptor-interacting protein 1 (RIP1) death domain and regulates caspase 8-dependent nuclear factor κB (NF-κB) activation. J Biol Chem 289:3876–3887
Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P (2000) µ1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J 19:2193–2203
Mindell JA (2012) Lysosomal acidification mechanisms. Ann Rev Physiol 74:69–86
Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G (1995) Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol 131:1715–1726
Nakatsu F, Hase K, Ohno H (2014) The role of clathrin adaptor AP-1: polarized sorting and beyond. Membranes 4:747–763
Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J (2004) Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol 164:1065–1076
Pérez-Victoria FJ, Mardones GA, Bonifacino JS (2008) Requirement of the human GARP complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol Biol Cell 19:2350–2362
Pfeffer SR (2011) Entry at the trans-face of the Golgi. Cold Spring Harb Perspect Biol 3:a005272
Pols MS, Klumperman J (2009) Trafficking and function of the tetraspanin CD63. Exp Cell Res 315:1584–1592
Pols MS, van Meel E, Oorschot V, ten Brink C, Fukuda M, Swetha MG, Mayor S, Klumperman J (2013) hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat Commun 4:1361
Progida C, Bakke O (2016) Bidirectional traffic between the Golgi and the endosomes—machineries and regulation. J Cell Sci 129:3971–3982
Röttger S, White J, Wandall HH, Olivo JC, Stark A, Bennett EP, Whitehouse C, Berger EG, Clausen H, Nilsson T (1998) Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J Cell Sci 111:45–60
Royle SJ (2006) The cellular functions of clathrin. Cell Mol Life Sci 63:1823–1832
Ruvio R, Anne C, Sagné C, Gasnier B (2009) Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim Biophys Acta 1793:636–649
Saftig P, Klumperman J (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol 10:623–635
Schröder J, Lüllmann-Rauch R, Himmerkus N, Pleines I, Nieswandt B, Orinska Z, Koch-Nolte F, Schröder B, Bleich M, Saftig P (2009) Deficiency of the tetraspanin CD63 associated with kidney pathology but normal lysosomal function. Mol Cell Biol 29:1083–1094
Schröder BA, Wrocklage C, Hasilik A, Saftig P (2010) The proteome of lysosomes. Proteomics 10:4053–4076
Schwake M, Schröder B, Saftig P (2013) Lysosomal membrane proteins and their central role in physiology. Traffic 14:739–748
Scott CC, Vacca F, Gruenberg J (2014) Endosome maturation, transport and functions. Semin Cell Dev Biol 31:2–10
Shinkai Y, Yoshida MC, Maeda K, Kobata T, Maruyama K, Yodoi J, Yagita H, Okumura K (1989) Molecular cloning and chromosomal assignment of a human perforin (PFP) gene. Immunogenetics 30:452–457
Staudt C, Puissant E, Boonen M (2017) Subcellular trafficking of mammalian lysosomal proteins: an extended view. Int J Mol Sci 18:47
Storrie B, White J, Röttger S, Stelzer EHK, Suganuma T, Nilsson T (1998) Recycling of Golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol 143:1505–1521
Ward JM, Reynolds CW (1983) Large granular lymphocyte leukemia. A heterogeneous lymphocytic leukemia in F344 rats. Am J Pathol 111:1–10
Wettey FR, Hawkins SFC, Stewart A, Luzio JP, Howard JC, Jackson AP (2002) Controlled elimination of clathrin heavy-chain expression in DT40 lymphocytes. Science 297:1521–1525
Xu X, Nagarajan H, Lewis NE, Pan S, Cai Z, Lin X, Chen W, Xie M, Wang W, Hammond S, Andersen MR, Neff N, Passarelli B, Koh W, Fan HC, Wang J, Gui Y, Lee KH, Betenbaugh MJ, Quake SR, Famili I, Palsson BO, Wang J (2011) The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat Biotech 29:735–741
Acknowledgements
We are very grateful to Drs. Della Reynolds, Craig W. Reynolds, Hideo Yagita, and Rafick-Pierre Sékaly for their kind gifts. We also thank Yoko Yaji and Hisashi Yamada for their contribution to this study. This work was partly supported by JSPS KAKENHI Grant numbers 22658036, 15K14920, and 19H02885 (to T.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baba, K., Kuwada, S., Nakao, A. et al. Different localization of lysosomal-associated membrane protein 1 (LAMP1) in mammalian cultured cell lines. Histochem Cell Biol 153, 199–213 (2020). https://doi.org/10.1007/s00418-019-01842-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-019-01842-z