Abstract
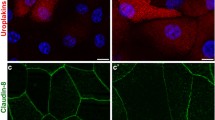
Superficial cell desquamation followed by differentiation of newly exposed superficial cells induces regeneration of the urinary bladder epithelium, urothelium. In the present work, chitosan was evaluated as a new inducer of urothelial cell desquamation, in order to study the regeneration of mouse urothelial cells in vivo. Intravesical application of chitosan dispersion caused complete removal of only the superficial layer of cells within 20 min of treatment. Differentiation of the new superficial layer was followed by the appearance and distribution of three urothelial differentiation markers, tight junction protein ZO1, cytokeratin 20 and the maturation of the apical plasma membrane. The arrangement of ZO1 into continuous lines in individual cells of the intermediate layer was already found after 10 min of chitosan application, when desquamation had just started. The appearance of the apical membrane changed from microvillar to typically scalloped within 20 min of regeneration, while complete arrangement of the cytokeratin 20 network took 60 min. These findings provide a new perspective on the rate of the differentiation process in the urothelium and make chitosan a new and a very controllable tool for studies on urothelial regeneration.






Similar content being viewed by others
References
Acharya P, Beckel J, Ruiz WG, Wang E, Rojas R, Birder L, Apodaca G (2004) Distribution of the tight junction proteins ZO-1, occludin, and claudin-4, -8, and -12 in bladder epithelium. Am J Physiol Renal Physiol 287:F305–F318
Aronson M, Medalia O, Amichay D, Nativ O (1988) Endotoxin-induced shedding of viable uroepithelial cells is an antimicrobial defense mechanism. Infect Immun 56:1615–1617
Artursson P, Lindmark T, Davis SS, Illum L (1994) Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res 11:1358–1361
Baskin L, Meaney D, Landsman A, Zderic SA, Macarak E (1994) Bovine bladder compliance increases with normal fetal development. J Urol 152:692–695
Baskin LS, Hayward SW, Young PF, Cunha GR (1996) Ontogeny of the rat bladder: smooth muscle and epithelial differentiation. Acta Anat (Basel) 155:163–171
Dalal E, Medalia O, Harari O, Aronson M (1994) Moderate stress protects female mice against bacterial infection of the bladder by eliciting uroepithelial shedding. Infect Immun 62:5505–5510
Erman A, Jezernik K, Stiblar-Martincic D, Romih R, Veranic P (2001) Postnatal restoration of the mouse urinary bladder urothelium. Histochem Cell Biol 115:309–316
Farsund T (1975) Cell kinetics of mouse urinary bladder epithelium. I. Circadian and age variations in cell proliferation and nuclear DNA content. Virchows Arch B Cell Pathol 18:35–49
Goldstein P, Kroemer G (2006) Cell death by necrosis: towards a molecular definition. TRENDS Biochem Sci 32:37–43
Hasegawa M, Yagi K, Iwakawa S, Hirai M (2001) Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn J Cancer Res 92:459–466
Hejazi R, Amiji M (2003) Chitosan-based gastrointestinal delivery systems. J Control Release 89:151–165
Hu P, Meyers S, Liang FX, Deng FM, Kachar B, Zeidel ML, Sun TT (2002) Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. Am J Physiol Renal Physiol 283:F1200–F1207
Jacob J, Hindmarsh JR, Ludgate CM, Chisholm GD (1982) Observations on the ultrastructure of human urothelium: the response of normal bladder of elderly subjects to hyperthermia. Urol Res 10:227–237
Jenings CD, Boleyn K, Bridges SR, Wood PJ, Anderson JW (1988) A comparison of the lipid-lowering and intestinal morphological effects of cholestyramine, chitosan, and oat gum in rats. Proc Soc Exp Biol Med 189:13–20
Jost SP (1989) Cell cycle of normal bladder urothelium in developing and adult mice. Virchows Arch B Cell Pathol Incl Mol Pathol 57:27–36
Kachar B, Liang F, Lins U, Ding M, Wu XR, Stoffler D, Aebi U, Sun TT (1999) Three-dimensional analysis of the 16 nm urothelial plaque particle: luminal surface exposure, preferential head-to-head interaction, and hinge formation. J Mol Biol 15:595–608
Kerec M, Bogataj M, Veranic P, Mrhar A (2005) Permeability of pig urinary bladder wall: the effect of chitosan and the role of calcium. Eur J Pharm Sci 25:113–121
Kos MK, Bogataj M, Veranic P, Mrhar A (2006) Permeability of pig urinary bladder wall: time and concentration dependent effect of chitosan. Biol Pharm Bull 29:1685–1691
Koss LG (1967) A light and electron microscopic study of the effects of a single dose of cyclophosphamide on various organs in the rat. I. The urinary bladder. Lab Invest 16:44–65
Kotzé AF, Luessen HL, de Leeuw BJ, de Boer BG, Verhoef JC, Junginger HE (1997) N-Trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: in vitro evaluation in intestinal epithelial cells (Caco-2). Pharm Res 14:1197–1202
Kreft ME, Sterle M, Veranic P, Jezernik K (2005) Urothelial injuries and the early wound healing response: tight junctions and urothelial cytodifferentiation. Histochem Cell Biol 123:529–539
Kunze E, Wöltjen HH, Nehm FJ, Schauer A (1981) Cell cycle kinetics of regenerating urothelial cells of the rat urinary bladder after partial cystectomy. Virchows Arch B Cell Pathol Incl Mol Pathol 38:117–125
Levi PE, Cooper EH, Anderson CK, Williams RE (1969) Analyses of DNA content, nuclear size and cell proliferation of transitional cell carcinoma in man. Cancer 23:1074–1085
Locher GW, Cooper EH (1970) Repair of rat urinary bladder epithelium following injury by cyclophosphamide. Invest Urol 8:116–123
Luna EJ, Hitt AL (1992) Cytoskeleton-plasma membrane interactions. Science 258:955–964 Review
Messier B, Leblond CP (1960) Cell proliferation and migration as revealed by adioautography after injection of thymidine-H3 into male rats and mice. Am J Anat 106:247–285
Miura T, Usami M, Tsuura Y, Ishida H, Seino Y (1995) Hypoglycemic and hypolipidemic effect of chitosan in normal and neonatal streptozotocin-induced diabetic mice. Biol Pharm Bull 18:1623–1625
Moll R, Wu XR, Lin JH, Sun TT (1995) Uroplakins, specific membrane proteins of urothelial umbrella cells, as histological markers of metastatic transitional cell carcinomas. Am J Pathol 147:1383–1397
Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ (1998) Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497
Mulvey MA, Schilling JD, Hultgren SJ (2001) Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 69:4572–4579
Nativ O, Dalal E, Hidas G, Aronson M (2006) EDTA-induced urothelial cell shedding for the treatment of superficial bladder cancer in the mouse. Int J Urol 13:1344–1346
Philips FS, Sternberg SS, Cronin AP, Vidal PM (1961) Cyclophosphamide and urinary bladder toxicity. Cancer Res 21:1577–1589
Ranaldi G, Marigliano I, Vespignani I, Perozzi G, Sambuy Y (2002) The effect of chitosan and other polycations on tight junction permeability in the human intestinal Caco-2 cell line. J Nutr Biochem 13:157–167
Romih R, Jezernik K, Masera A (1998) Uroplakins and cytokeratins in the regenerating rat urothelium after sodium saccharin treatment. Histochem Cell Biol 109:263–269
Romih R, Veranic P, Jezernik K (1999) Actin filaments during terminal differentiation of urothelial cells in the rat urinary bladder. Histochem Cell Biol 112:375–380
Romih R, Koprivec D, Martincic DS, Jezernik K (2001) Restoration of the rat urothelium after cyclophosphamide treatment. Cell Biol Int 25:531–537
Şenel S, Hincal AA (2001) Drug permeation enhancement via buccal route: possibilities and limitations. J Control Release 72:133–144
Stewart FA (1986) Mechanism of bladder damage and repair after treatment with radiation and cytostatic drugs. Br J Cancer Suppl 7:280–291
Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, Zeidel M, Stoffer D, Apodaca G (2002) Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell 13:830–846
Veranic P, Jezernik K (2000) The response of junctional complexes to induced desquamation in mouse bladder urothelium. Biol Cell 92:105–113
Veranic P, Jezernik K (2002) Trajectorial organisation of cytokeratins within the subapical region of umbrella cells. Cell Motil Cytoskeleton 53:317–325
Veranic P, Romih R, Jezernik K (2004) What determines differentiation of urothelial umbrella cells? Eur J Cell Biol 83:27–34
Wang Y, Chen M, Wolosin JM (1993) ZO-1 in corneal epithelium; stratal distribution and synthesis induction by outer cell removal. Exp Eye Res 57:283–292
Watanabe S, Sasaki J (1992) 12-O-Tetradecanoylphorbol-13-acetate induces selective desquamation of superficial cells in rat urinary bladder epithelium. Cell Tissue Res 268:239–245
Wolosin JM, Chen M (1993) Ontogeny of corneal epithelial tight junctions: stratal locale of biosynthetic activities. Invest Ophthalmol Vis Sci 34:2655–2664
Yu S, Zhao Y, Wu F, Zhang X, Lu W, Zhang H, Zhang Q (2004) Nasal insulin delivery in the chitosan solution: in vitro and in vivo studies. Int J Pharm 281:11–23
Acknowledgment
The authors thank Mr Martin Cregeen for checking the English of the text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veranič, P., Erman, A., Kerec-Kos, M. et al. Rapid differentiation of superficial urothelial cells after chitosan-induced desquamation. Histochem Cell Biol 131, 129–139 (2009). https://doi.org/10.1007/s00418-008-0492-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-008-0492-x