Abstract
Purpose
To clarify the abilities of circumpapillary retinal nerve fiber layer thickness (cpRNFLT) obtained by optical coherence tomography (OCT) and circumpapillary vessel density (cpVD) measured by OCT-angiography to distinguish different stages in primary open-angle glaucoma determined by 24–2 or 30–2 static visual field (VF) testing.
Methods
This retrospective study includes 25 healthy normal eyes of 25 subjects and 87 primary open-angle glaucoma eyes of 87 patients. Areas under the receiver operating characteristic curves (AUROC) were evaluated for determining glaucoma stages using cpRNFLT and cpVD. The absolute errors of the estimated mean total deviation (mTD) using optimal models with cpRNFLT and cpVD were also compared.
Results
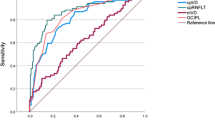
The AUROCs for discriminating glaucomatous eyes from normal eyes was significantly higher for cpRNFLT than the respective AUROCs for cpVD (0.969 [95% CI 0.939 to 0.998] vs. 0.872 [95% CI 0.806 to 0.938], p = 0.006), whereas cpVD had significantly higher AUROC for discriminating severe glaucoma eyes from moderate glaucoma eyes than cpRNFLT (0.771 [95% CI 0.655 to 0.886] vs. 0.578 [95% CI 0.420 to 0.736], p = 0.022). The mean absolute error in estimating mTD using both cpRNFLT and cpVD was significantly less than the error using cpRNFLT alone (4.56 ± 3.76 dB vs. 5.39 ± 4.00 dB, p = 0.027).
Conclusion
Our results suggest that cpVD is better for follow-ups after moderate stage. The combination of cpRNFLT and cpVD may improve VF estimation compared to cpRNFLT alone.




Similar content being viewed by others
References
Altangerel U, Spaeth GL, Rhee DJ (2003) Visual function, disability, and psychological impact of glaucoma. Curr Opin Ophthalmol 14:100–105. https://doi.org/10.1097/00055735-200304000-00009
Flammer J, Drance SM, Zulauf M (1984) Differential light threshold: short-and long-term fluctuation in patients with glaucoma, normal controls, and patients with suspected glaucoma. Arch Ophthalmol 102:704–706
Fujino Y, Murata H, Matsuura M et al (2018) Mapping the Central 10° Visual Field to the Optic Nerve Head Using the Structure-Function Relationship. Invest Ophthalmol Vis Sci 59:2801–2807. https://doi.org/10.1167/iovs.17-23485
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA (2000) Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 107:1809–1815. https://doi.org/10.1016/s0161-6420(00)00284-0
Malik R, Swanson WH, Garway-Heath DF (2012) ‘Structure–function relationship’ in glaucoma: past thinking and current concepts. Clin Exp Ophthalmol 40:369–380. https://doi.org/10.1111/j.1442-9071.2012.02770.x
Mwanza J-C, Budenz DL, Warren JL, Webel AD, Reynolds CE, Barbosa DT, Lin S (2015) Retinal nerve fibre layer thickness floor and corresponding functional loss in glaucoma. Br J Ophthalmol 99:732–737. https://doi.org/10.1136/bjophthalmol-2014-305745
Blumenthal EZ, Horani A, Sasikumar R, Garudadri C, Udaykumar A, Thomas R (2006) Correlating structure with function in end-stage glaucoma. Ophthalmic Surg Lasers Imaging 37:218–223. https://doi.org/10.3928/15428877-20060501-06
Hood DC, Kardon RH (2007) A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res 26:688–710. https://doi.org/10.1016/j.preteyeres.2007.08.001
Yarmohammadi A, Zangwill LM, Diniz-Filho A et al (2016) Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 123:2498–2508. https://doi.org/10.1016/j.ophtha.2016.08.041
Moghimi S, Bowd C, Zangwill LM et al (2019) Measurement floors and dynamic ranges of OCT and OCT angiography in glaucoma. Ophthalmology 126:980–988. https://doi.org/10.1016/j.ophtha.2019.03.003
Phillips MJ, Dinh-Dang D, Bolo K et al (2021) Steps to measurement floor of an optical microangiography device in glaucoma. Am J Ophthalmol 231:58–69. https://doi.org/10.1016/j.ajo.2021.05.012
Chung JK, Hwang YH, Wi JM, Kim M, Jung JJ (2017) Glaucoma diagnostic ability of the optical coherence tomography angiography vessel density parameters. Curr Eye Res 42:1458–1467. https://doi.org/10.1080/02713683.2017.1337157
Geyman LS, Garg RA, Suwan Y et al (2017) Peripapillary perfused capillary density in primary open<strong>-</strong>angle glaucoma across disease stage<strong>:</strong> an optical coherence tomography angiography study. Br J Ophthalmol 101:1261–1268. https://doi.org/10.1136/bjophthalmol-2016-309642
Akiyama K, Saito H, Shirato S et al (2022) Diagnostic ability and sectoral structure–function relationship of circumpapillary and macular superficial vessel density in early glaucomatous eyes. Sci Rep 12:5991. https://doi.org/10.1038/s41598-022-10033-1
Yu HH, Maetschke SR, Antony BJ, Ishikawa H, Wollstein G, Schuman JS, Garnavi R (2021) Estimating global visual field indices in glaucoma by combining macula and optic disc OCT scans using 3-dimensional convolutional neural networks. Ophthalmol Glaucoma 4:102–112. https://doi.org/10.1016/j.ogla.2020.07.002
Xu C, Saini C, Wang M et al (2023) Combined Model of OCT Angiography and Structural OCT Parameters to Predict Paracentral Visual Field Loss in Primary Open-Angle Glaucoma. Ophthalmol Glaucoma 6:255–265. https://doi.org/10.1016/j.ogla.2022.10.001
Cohen JF, Korevaar DA, Altman DG et al (2016) STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 6:e012799. https://doi.org/10.1136/bmjopen-2016-012799
Wang X, Chen J, Kong X, Sun X (2020) Immediate changes in peripapillary retinal vasculature after intraocular pressure elevation -an optical coherence tomography angiography study. Curr Eye Res 45:749–756. https://doi.org/10.1080/02713683.2019.1695843
Holló G (2017) Influence of large intraocular pressure reduction on peripapillary OCT vessel density in ocular hypertensive and glaucoma eyes. J Glaucoma 26:e7–e10. https://doi.org/10.1097/ijg.0000000000000527
Hong KL, Burkemper B, Urrea AL et al (2021) Hemiretinal asymmetry in peripapillary vessel density in healthy, glaucoma suspect, and glaucoma eyes. Am J Ophthalmol 230:156–165. https://doi.org/10.1016/j.ajo.2021.05.019
WuDunn D, Takusagawa HL, Sit AJ et al (2021) OCT angiography for the diagnosis of glaucoma: A report by the American Academy of Ophthalmology. Ophthalmology 128:1222–1235. https://doi.org/10.1016/j.ophtha.2020.12.027
Yarmohammadi A, Zangwill LM, Diniz-Filho A et al (2016) Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci 57:451–459. https://doi.org/10.1167/iovs.15-18944
Rao HL, Pradhan ZS, Weinreb RN et al (2017) A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS ONE 12:e0173930. https://doi.org/10.1371/journal.pone.0173930
Lee EJ, Lee KM, Lee SH, Kim TW (2016) OCT angiography of the peripapillary retina in primary open-angle glaucoma. Invest Ophthalmol Vis Sci 57:6265–6270. https://doi.org/10.1167/iovs.16-20287
Sihota R, Sony P, Gupta V, Dada T, Singh R (2006) Diagnostic capability of optical coherence tomography in evaluating the degree of glaucomatous retinal nerve fiber damage. Invest Ophthalmol Vis Sci 47:2006–2010. https://doi.org/10.1167/iovs.05-1102
Kuroda F, Iwase T, Yamamoto K, Ra E, Terasaki H (2020) Correlation between blood flow on optic nerve head and structural and functional changes in eyes with glaucoma. Sci Rep 10:729. https://doi.org/10.1038/s41598-020-57583-w
Zhu H, Crabb DP, Schlottmann PG et al (2010) Predicting visual function from the measurements of retinal nerve fiber layer structure. Invest Ophthalmol Vis Sci 51:5657–5666. https://doi.org/10.1167/iovs.10-5239
Christopher M, Bowd C, Belghith A et al (2020) Deep learning approaches predict glaucomatous visual field damage from OCT optic nerve head en face images and retinal nerve fiber layer thickness maps. Ophthalmology 127:346–356. https://doi.org/10.1016/j.ophtha.2019.09.036
Bogunović H, Kwon YH, Rashid A et al (2015) Relationships of retinal structure and humphrey 24–2 visual field thresholds in patients with glaucoma. Invest Ophthalmol Vis Sci 56:259–271. https://doi.org/10.1167/iovs.14-15885
Hashimoto Y, Asaoka R, Kiwaki T et al (2021) Deep learning model to predict visual field in central 10° from optical coherence tomography measurement in glaucoma. Br J Ophthalmol 105:507–513. https://doi.org/10.1136/bjophthalmol-2019-315600
Wong D, Chua J, Tan B et al (2021) Combining OCT and OCTA for Focal Structure-Function Modeling in Early Primary Open-Angle Glaucoma. Invest Ophthalmol Vis Sci 62:8–8. https://doi.org/10.1167/iovs.62.15.8
Shin JW, Kwon J, Lee J, Kook MS (2019) Relationship between vessel density and visual field sensitivity in glaucomatous eyes with high myopia. Br J Ophthalmol 103:585–591. https://doi.org/10.1136/bjophthalmol-2018-312085
Acknowledgements
The swept source optical coherence tomography device used in this study was on loan from Carl Zeiss Meditec.
Funding
This work was supported by a Grant-in-Aid for Young Scientists (R.T., number 21K16870) from JSPS KAKENHI (http://www.jsps.go.jp/).
Author information
Authors and Affiliations
Contributions
KY, RT, TI and KN were involved in the design and conduct of the study; KY, RT, and TI were involved in the collection, management, analysis, and interpretation of data; and KY, RT, KK, RA, HT, TI, and KN were involved in the preparation, review, and approval of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was conducted in adherence to the tenets of the Declaration of Helsinki. The procedures were approved by the Institutional Review Board and the Ethics Committee of the Nagoya University Graduate School of Medicine.
Informed consent
The institutional review board exempted this study from informed consent due to the retrospective study design. We published the study protocol on the website and offered participants the opportunity to opt out.
Standards of reporting
The Standards for Reporting of Diagnostic Accuracy Studies (STARD) checklist for this study is shown as a supplementary information [17].
Competing interests
KY, None; RT, None; YK, None; KK received honoraria for lectures from Senju; RA, None; HT received non-financial research support from Carl Zeiss related to swept source optical coherence tomography and grants and honoraria for lectures from Otsuka, Kowa, Santen, Senju, Sanofi, Alcon, Novartis, ROHTO, and Wakamoto. She received grants and consulting fees from Bayer and honoraria for lectures from HOYA and Johnson & Johnson; TI, None; KN received grants from Takara bio, Takeda pharmaceutical, JCR Pharma, Alcon, and Bayer, and honoraria for lectures from Novartis, Santen, Chugai Pharma, Kowa, Senju, Otsuka, and Wakamoto. He has a patent related to a single AAV vector.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamaguchi, K., Tomita, R., Koyanagi, Y. et al. Abilities of circumpapillary retinal nerve fiber layer thickness and vascular density to discriminate stages in primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol 262, 1221–1229 (2024). https://doi.org/10.1007/s00417-023-06302-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06302-y