Abstract
Purpose
Osteogenesis imperfecta (OI) is a rare inherited disease affecting collagen-rich tissues. Ocular complications have been reported such as thin corneas, low ocular rigidity, keratoconus, among others. The purpose of this study is to characterize corneal tomographic features in OI patients compared to unaffected patients, with particular focus on commonly studied keratoconus indices.
Methods
Cross-sectional case–control study including 37 OI patients and 37 age-matched controls. Patients and controls underwent comprehensive ophthalmological examination including corneal Scheimpflug tomography with a Pentacam HR device (Oculus Optikgeräte GmbH, Wetzlar, Germany) to analyse and compare topometric, tomographic, pachymetric and Belin-Ambrósio Enhanced Ectasia Display III (BAD-D) data of both eyes of each patient.
Results
Most OI patients had type I disease (n = 24; 65%) but type III–VII patients were also included. Two patients had clinically overt bilateral keratoconus. OI patients had significantly higher maximum keratometry (45.2 ± 2.1 vs. 43.7 ± 1.2; p = 0.0416), front and back elevation (3.0 ± 3.3 vs. 2.1 ± 1.3, p = 0.0201; 11.1 ± 8.2 vs. 5.0 ± 3.7, p < 0.0001), index of surface variance (25.5 ± 13 vs. 17.4 ± 8.3; p = 0.0016), index of vertical asymmetry (0.21 ± 0.14 vs. 0.15 ± 0.06; p = 0.0215), index of height asymmetry (9.2 ± 14 vs. 6.0 ± 4.5; p = 0.0421), index of height decentration (0.02 ± 0.01 vs. 0.01 ± 0.01; p < 0.0001) and average pachymetric progression (1.01 ± 0.19 vs. 0.88 ± 0.14; p < 0.0001) readings. Thinnest corneal thickness and maximum Ambrósio relational thickness were significantly lower (477 ± 52 vs. 543 ± 26; 387 ± 95 vs. 509 ± 49; p < 0.0001). Two-thirds of OI patients had corneas with a minimum thickness < 500 µm. BAD-D value was significantly higher in OI patients (2.1 ± 1.4 vs. 0.9 ± 0.2; p < 0.0001).
Conclusion
OI patients showed significant changes in corneal profiles compared with healthy subjects. A high proportion of patients had tomographically suspect corneas when using keratoconus diagnostic indices. Further studies are warranted to assess the true risk of corneal ectasia in OI patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.

Introduction
Osteogenesis imperfecta (OI) is a rare inherited connective tissue disease affecting collagen production, with a prevalence around 6–7:100,000, commonly caused by mutations in the COL1A1 and COL1A2 genes, which encode α1 and α2 chains of type I collagen respectively, with a predominantly autosomal dominant inheritance pattern [1]. Several clinical forms of OI have been described according to early lethality, onset and number of fractures, skeletal deformity, patient stature, radiological and other clinical findings. Classically, OI patients have been classified into OI types I–IV according to Sillence [1,2,3]. OI type I (“non-deforming OI with blue sclerae”), the most common and mildest clinical form, is generally caused by COL1A1 or COL1A2 mutations determining a quantitative deficit of collagen type I, with patients suffering multiple fractures during childhood up to late puberty. Type II OI is severe and almost always lethal perinatally. OI type III is associated a qualitative collagen defect, with more numerous and severe fractures in childhood than type I, progressive deformation, and a wider variety of causative genetic mutations. OI type IV (“common variable OI with normal sclerae”) is the second most prevalent type and displays a clinical picture similar to OI type I but several genetic loci have been implicated. With the advent of widespread genetic testing and wide-genome assessments, more genetic defects in different targets pertaining to collagen metabolism have been identified as causative, which have expanded the original Sillence classification to include novel and rarer OI forms recognised by the Online Mendelian Inheritance in Man (OMIM) database [4,5,6,7]. These rarer forms include OI type V (OMIM #610,967; autosomal dominant OI with calcification in interosseous membranes, caused by IFITM5 mutations), type VI (OMIM #613,982, autosomal recessive, similar to type IV but usually more severe), type VII (OMIM #610,682, autosomal recessive severe and progressively deforming), among others.
Patients with OI can display several multisystem manifestations. The classic ocular sign of OI is a blueish-grey discoloration of the sclerae, particularly in type I disease [8]. However, other ocular manifestations have been reported, such as absent or atrophic Bowman’s layer, keratoconus (KC), high myopia, glaucoma, low ocular rigidity, reduced corneal hysteresis, increased risk of scleral rupture, retinal haemorrhages, subretinal and choroidal neovascularization, among others [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Corneal thinning has been recently highlighted as a possible hallmark of the disease, particularly in OI type I [10, 24]. Most of these changes seem to empirically stem from a connective tissue defect and higher tissue fragility, given that the corneoscleral layer contains predominantly type I collagen [30,31,32]. However, the risk of these manifestations in OI patients has not been well studied and most associations arise from case reports and small series, including in the case of KC [9, 33, 34]. Although clinical KC diagnosis with direct observation at the slit-lamp is possible, particularly in advanced cases, corneal tomographic analysis is currently the screening, diagnostic and progression analysis tool of choice [35,36,37].
The present study aims to characterize corneal tomographic features in OI patients compared to unaffected patients, with particular focus on commonly used and clinically validated KC diagnostic indices.
Materials and methods
Design and population
A cross-sectional observational case–control study was undertaken from 2019 to 2022 in Hospital Santa Maria, CHULN, Lisbon, Portugal. Thirty-seven (37) Portuguese adult patients with OI diagnosis and 37 age-matched controls were included in this study. All patients included had an OI diagnosis confirmed by a medical genetics expert based on a compatible clinical, each with a lifelong history of multiple and recurring low trauma fractures and compatible radiological findings. Disease classification was made from the Skeletal Dysplasia Nomenclature Group [3] and the Online Mendelian Inheritance in Man (OMIM) database [6] according to clinical and genetic findings. Exclusion criteria were age < 18 years, uncertain diagnosis, previous corneal surgery or trauma. All control patients included were healthy subjects who came in for routine clinical observation and had normal ophthalmological examinations other than refractive error within 1.0 dioptre (D) of spherical equivalent. Approval was obtained from the CHULN/CAML ethics committee and this study adhered to the tenets of the Declaration of Helsinki.
Ophthalmological evaluation
Patients underwent ophthalmological evaluation, including autorefraction, intraocular pressure (IOP) measurement using Goldmann applanation tonometry (GAT), slit-lamp biomicroscopy and mydriatic fundus examination. Patient and control eyes also underwent corneal Scheimpflug tomography using a Pentacam HR device (Oculus Optikgeräte GmbH, Wetzlar, Germany). Patients wearing contact lenses were instructed to discontinue its use before undergoing corneal tomography (for at least 2 weeks for rigid gas-permeable lenses and 48 h for soft lenses). Topographic, tomographic and pachymetric parameters included in this analysis included mean front and maximum keratometry (Km and Kmax respectively), net power of corneal astigmatism (Ast), thinnest corneal thickness (CTmin), anterior and posterior surface elevation at the thinnest point in relation to a 8-mm best-fit sphere (FEle and BEle respectively), index of surface variance (ISV), index of vertical asymmetry (IVA), index of height asymmetry (IHA), index of height decentration (IHD), average pachymetric progression index (PPIavg), maximum Ambrósio relational thickness (ARTmax) and Belin–Ambrósio Enhanced Ectasia Display III software-generated D score for deviation from normality (BAD-D). Pertinent clinical data was retrieved from patient files.
Statistical analysis
This study was powered to detect a clinically relevant difference in BAD-D and thinnest corneal thickness. A minimum sample size of 34 subjects in each group would be required to detect a 1-point change in BAD-D and 50 µm in thinnest corneal thickness, assuming a mean ± standard deviation of 0.96 ± 0.8 for BAD-D and 539 ± 31 µm for corneal thickness, as reported in previous studies [35, 38], with a 2-sided significance level of 0.003 and 99% confidence level [39].
Statistical analysis was performed with GraphPad Prism 9 (version 9.5.0, San Diego, CA, USA). Kolmogorov–Smirnov and Shapiro–Wilk tests were used to assess distribution normality. Chi-square test was used for categorical variables, whereas Student t and Kolmogorov–Smirnov tests were used for parametric and non-parametric quantitative data analysis, respectively. Pearson and Spearman correlation analyses were performed between variables with a normal or non-normal distribution, respectively. A p-value < 0.05 was used to assert statistical significance.
Results
Both eyes of thirty-seven (37) OI patients and 37 age-matched controls were included in this analysis. Age distribution was similar between OI and controls (p = 0.8467) as was female to male ratio (30:7 in the OI group and 29:8 in the control group). Of the 37 OI patients, 24 (65%) were classified as OI type I, most of whom had quantitative COL1A1 mutations (23/24). The remaining 13 patients had qualitative mutations and were classified as OI type III (n = 3, 8%), type IV (n = 7, 19%), type V (n = 1, 3%), type VI (n = 1, 3%) and type VII (n = 1, 3%). All OI type I and type III patients displayed blue sclerae bilaterally, a significantly higher proportion than in the type IV–VII subgroup (n = 27 vs. n = 1; p < 0.0001). All patients but one in each group (OI and controls) were phakic, and there was no other surgical history reported. Two OI type I patients had a previous diagnosis of bilateral keratoconus, with compatible clinical signs at the slit-lamp and used rigid gas-permeable contact lenses as a visual aid. No other patient used contact lenses. One OI patient had a history Behçet’s disease complicated with previous posterior uveitis and uveitic glaucoma under topical medication. No patient in either group (OI or control) had history of atopic disease such as allergies, asthma or eczema, or reported history of eye-rubbing behaviour or corneal microtrauma, including those with diagnosed KC. Regarding familiarity, patients 29 and 30 were siblings, and patients 3 and 2 were mother and son. No other familial relationship was recorded. Clinical classification, mutated genes and overall ocular findings in OI patients are detailed in Table 1.
All comparisons except mean keratometry (Km, 43.6 ± 1.9 vs. 43.7 ± 1.2, p = 0.6330) showed significant differences between groups (Table 2). Spherical and cylindrical errors, true net corneal astigmatism power and Kmax were all higher in OI patients versus controls (1.6 ± 5.1 vs. 0.2 ± 0.5; 1.3 ± 0.9 vs. 0.4 ± 0.2; 1.5 ± 0.8 vs. 0.3 ± 0.2; 45.2 ± 2.1 vs. 43.7 ± 1.2; p < 0.05 for all comparisons). Elevation maps showed higher front and back elevation at thinnest point in OI patients (3.0 ± 3.3 vs. 2.1 ± 1.3, p = 0.0201; 11.1 ± 8.2 vs. 5.0 ± 3.7, p < 0.0001). ISV, IVA, IHA and IHD were also higher in OI patients (p < 0.05 for all; see Table 2). Pachymetric measurements such as thinnest corneal thickness and ARTmax were significantly lower (477 ± 52 vs. 543 ± 26; 387 ± 95 vs. 509 ± 49; p < 0.0001 for both) whereas PPIavg was higher in OI patients (1.01 ± 0.19 vs. 0.88 ± 0.14; p < 0.0001). The proportion of OI patient eyes with thinnest pachymetry < 500 µm was significantly higher than in controls (n = 49 vs. 2; 66.2% vs. 2.7%; p < 0.0001). The BAD final D value was significantly higher in OI patients (2.1 ± 1.4 vs. 0.9 ± 0.2; p < 0.0001).
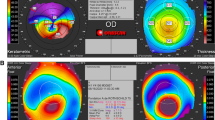
When comparing the same indices of OI patients classified as type I with patients with other clinical forms, OI type I patients showed significantly lower thinnest corneal thickness, ARTmax and BAD-D (p < 0.005 for all comparisons; Table 2), with all remaining parameters being similar between both groups. The proportion of thinnest pachymetry < 500 µm was also significantly higher in OI type I patients versus other OI types (n = 41 vs. 8; 85.4% vs. 33.3%; p < 0.0001). Both patients with previously diagnosed bilateral KC showing overt clinical signs had OI type I disease (patients 9 and 14, Table 1). Both displayed nipple-shaped central cones (base < 5 mm and Kmax within the central 3 mm zone [40, 41]). Their inter-eye BAD-D average was 7.32 and 5.9, Km was 48.4 and 40.8D, Kmax 50.4 and 43.2D, PPIavg 1.5 and 1.1, ARTmax 216 and 275, front elevation 14.5 and 13.5 µm, back elevation was 43.5 and 33 µm, and minimum pachymetry was 456 and 390 µm. The right eye Belin-Ambrósio Enhanced Ectasia Display for both patients is shown in Figs. 1 and 2. We detected no other case of corneal ectasia with clinical signs at the slit-lamp.
IOP was significantly lower in OI patients versus controls (13.9 ± 4.6 vs. 16.5 ± 5.0 mmHg; p < 0.0001) and correlated with corneal thickness (rs = 0.6226; p < 0.0001). A best-fit line using linear regression is showed in Fig. 3 to graphically display this relationship.
Discussion
KC is a progressive corneal ectasia with a variably reported prevalence ranging from a worldwide 1.38:1000 prevalence to as high as 47:1000 in some populations, and is linked to allergy, asthma, eczema and eye rubbing behaviour [42, 43]. Corneal tomography is the methodology of choice for screening and early diagnosis of KC through the use of several indices [35,36,37]. The BAD-D index is commonly used in clinical practice and has been shown to be one of the most sensitive and specific indices in KC screening, with proposed cut-offs of 1.34–1.66 for suspect corneas and 2.38–2.69 for definite diagnosis [35,36,37,38].
Our sample included 74 eyes from 37 OI patients and the same number of control eyes and patients. Our results indicate a significant deviation from healthy subject readings on almost all parameters measured in OI patients. All these changes in OI patients skewed from normality towards readings associated with tomographic criteria used for ectasia diagnosis [35,36,37]. A recent study from Keles et al. comparing 17 OI patients with a control group showed a similar trend of tomographic changes in OI patients [20]. Arbitrarily considering BAD-D cut-offs of 1.6 and 2.4 for suspect corneas and definite keratoconus diagnosis, 47 eyes (64%) from 28 patients (9 unilateral) would be considered as having suspect corneas, and 22 eyes (30%) from 11 patients (2 unilateral) would be classified as having definite keratoconus, while in the healthy subject group no case was recorded with a BAD-D over these cut-off values. The proportion of eyes with a BAD-D over 1.6 and 2.4 in the OI type I subgroup was 71% (n = 34) and 35% (n = 17) versus 46% (n = 12) and 12% (n = 3) in the remaining OI clinical forms. This may be partly explained by the fact that BAD-D is a composite index representing an overall deviation from normality combining data from several tomographic parameters including elevation data, keratometry readings and pachymetric data. Approximately two-thirds of OI patients showed thin corneas < 500 µm compared with less than 3% of eyes in normal patients, in line with previously published reports [19, 20]. The thinner corneas in OI patients may skew the BAD-D to higher readings limiting its usefulness in screening for keratoconus in this population. Therefore, the authors believe BAD-D should not be used alone for this purpose in patients with OI, in agreement with other authors [24]. However, the clinical usefulness (or lack thereof) of this parameter has not been demonstrated in this patient population.
Our study also found that OI type I patients had significantly lower corneal thickness and higher proportion of blue sclerae than eyes of patients with other OI types. This is in line with findings by Evereklioglu et al. who determined a correlation between reduced corneal thickness and the presence of blue sclerae in OI patients, which is also much more prevalent in OI type I patients [8,9,10]. This may suggest that overall corneoscleral layer thickness more predominantly reduced in these. However, to the authors’ knowledge, there have been no published reports on scleral thickness in OI patients.
We also report lower IOP readings in OI patients compared to controls and a correlation between IOP in corneal thickness in these patients, in line with previous studies [9, 22, 32]. It has been well established that corneal thickness correlates with IOP [44]. Additionally, studies have reported lower corneal hysteresis and resistance factor in OI patients, with lower tonometry IOP readings but higher corneal-compensated IOP versus controls [22, 32]. Glaucomatous structural and functional damage has been linked to lower corneal thickness and hysteresis [45,46,47] and COL1A1 mutations have been directly linked to glaucoma cases [48]. These findings may indicate a higher susceptibility of OI eyes for the development of glaucoma and suggest that corneal biomechanics should be assessed in OI patients. Despite few reports of glaucoma cases in OI patients, no comprehensive study has studied glaucoma risk in this population [21, 22, 25, 26].
The main limitations of this study include its cross-sectional design, preventing from having multiple timepoint measurements, which would be useful in monitoring corneal changes over time. Moreover, this cohort consists of only Portuguese patients with known cases of familiarity, which may not represent the wider OI population.
To the authors’ knowledge, this is one of the largest OI samples in the literature describing corneal tomographic profiles. Both other large publications with similar design and purpose had significantly younger populations which may have limited their ability to diagnose ectasia [20, 24]. This is the first OI cohort study to detail the corneal profiles of 2 cases of definite bilateral KC in OI type I patients. In conclusion, we found significant corneal topometric, tomographic and pachymetric changes in OI patients compared with healthy subjects. A significant proportion of OI patients displayed tomographically suspect corneas, and two patients had bilateral keratoconus. Further studies are warranted to determine the risk of keratoconus in OI patients, such as longitudinal analysis. Additionally, efforts should be made to assess the clinical validity of currently available keratoconus diagnostic indices in collagen-deficient thin corneas, such as those found in OI patients.
Data availability
Data are available upon request.
Code availability
Not applicable.
References
Steiner R, Basel D (2005) COL1A1/2 Osteogenesis Imperfecta. GeneReviews® [Internet]. University of Washington, Seattle, Seattle, pp 1993–2022
Sillence DO, Senn A, Danks DM (1979) Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 16:101–116
Bonafe L, Cormier-Daire V, Hall C et al (2015) Nosology and classification of genetic skeletal disorders: 2015 revision. Am J Med Genet Part A 167:2869–2892
Forlino A, Marini JC (2016) Osteogenesis imperfecta. Lancet 387:1657–1671
Marini JC, Reich A, Smith SM (2014) Osteogenesis imperfecta due to mutations in non-collagenous genes: lessons in the biology of bone formation. Curr Opin Pediatr 26:500–507
Robinson ME, Rauch F (2019) Mendelian bone fragility disorders. Bone 126:11–17
Thomas IH, DiMeglio LA (2016) Advances in the classification and treatment of osteogenesis imperfecta. Curr Osteoporos Rep 14:1–9
Sillence D, Butler B, Latham M et al (1993) Natural history of blue sclerae in osteogenesis imperfecta. Am J Med Genet 45:183–186
Evereklioglu C, Madenci E, Bayazit YA et al (2002) Central corneal thickness is lower in osteogenesis imperfecta and negatively correlates with the presence of blue sclera. Ophthalmic Physiol Opt 22:511–515
Hald JD, Folkestad L, Swan CZ et al (2018) Osteogenesis imperfecta and the teeth, eyes, and ears—a study of non-skeletal phenotypes in adults. Osteoporos Int 29:2781–2789
Scott A, Kashani S, Towler HMA (2005) Progressive myopia due to posterior staphyloma in type I Osteogenesis Imperfecta. Int Ophthalmol 26:167–169
Salcone EM, Hamdy S, Melki S et al (2014) Scleral perforations during routine traction test in a patient with osteogenesis imperfecta. J AAPOS 18:610–612
Oh EK, Choi HJ, Oh JY et al (2016) Sequential traumatic and spontaneous corneal rupture in patient with osteogenesis imperfecta. Can J Ophthalmol 51:e81–e84
Klug SE, Bek T (2017) Subretinal neovascularization as the only ocular sign of osteogenesis imperfecta: a case report. Acta Ophthalmol 95:e159–e160
Campagna G, Al-Mohtaseb Z, Khandelwal S et al (2018) Sequential traumatic corneal open globe rupture in a patient with osteogenesis imperfecta type I. Am J Ophthalmol Case Reports 11:35–36
Bellanca RF, Scarinci F, Parravano M (2018) Multimodal imaging in a young male with osteogenesis imperfecta complicated with choroidal neovascularization. Eur J Ophthalmol 30(1):NP21–NP24
Kobayashi A, Higashide T, Yokogawa H et al (2014) In vivo laser confocal microscopy findings of a cornea with osteogenesis imperfecta. Clin Ophthalmol 8:429–433
Treurniet S, Burger P, Ghyczy EAE et al (2022) Ocular characteristics and complications in patients with osteogenesis imperfecta: a systematic review. Acta Ophthalmol 100:e16–e28
Dimasi DP, Chen JY, Hewitt AW et al (2010) Novel quantitative trait loci for central corneal thickness identified by candidate gene analysis of osteogenesis imperfecta genes. Hum Genet 127:33–44
Keleş A, Doǧuizi S, Şahin NM et al (2020) Anterior segment findings in patients with osteogenesis imperfecta: a case-control study. Cornea 39:935–939
Rosbach J, Vossmerbaeumer U, Renieri G et al (2012) Osteogenesis imperfecta und Glaukom. Der Ophthalmol 109:479–482
Doolan E, O’Brien C (2021) Abnormal corneal properties in osteogenesis imperfecta and glaucoma: a case series. BMJ Open Ophthalmol 6:1–8
Pirouzian A, O’Halloran H, Scher C et al (2007) Traumatic and spontaneous scleral rupture and uveal prolapse in osteogenesis imperfecta. J Pediatr Ophthalmol Strabismus 44:315–317
Magalhaes OA, Rohenkohl HC, De Souza LT et al (2018) Collagen i defect corneal profiles in osteogenesis imperfecta. Cornea 37:1561–1565
Wallace DJ, Chau FY, Santiago-Turla C et al (2014) Osteogenesis imperfecta and primary open angle glaucoma: genotypic analysis of a new phenotypic association. Mol Vis 20:1174–1181
Alpogan O (2022) Association of osteogenesis imperfecta and glaucoma: case report. Ophthalmic Genet 1–5
Kaiser-Kupfer MI, McCain L, Shapiro JR et al (1981) Low ocular rigidity in patients with osteogenesis imperfecta. Invest Ophthalmol Vis Sci 20:807–809
Pedersen U, Bramsen T (1984) Central corneal thickness in osteogenesis imperfecta and otosclerosis. ORL 46:38–41
Ganesh A, Jenny C, Geyer J et al (2004) Retinal hemorrhages in type I osteogenesis imperfecta after minor trauma. Ophthalmology 111:1428–1431
Meek KM (2009) Corneal collagen-its role in maintaining corneal shape and transparency. Biophys Rev 1:83–93
Boote C, Sigal IA, Grytz R et al (2020) Scleral structure and biomechanics. Prog Retin Eye Res 74:100773
Lagrou LM, Gilbert J, Hannibal M et al (2018) Altered corneal biomechanical properties in children with osteogenesis imperfecta. J AAPOS 22:183-187.e1
Zeri F, Swann PG, Naroo S (2018) Osteogenesis imperfecta and keratoconus in an Italian family. Clin Exp Optom 101:400–403
Kwitko S, Pretto J (2017) Corneal cross-linking in a child with osteogenesis imperfecta syndrome and keratoconus. Australas Med J 10:567–570
Hashemi H, Beiranvand A, Yekta A et al (2016) Pentacam top indices for diagnosing subclinical and definite keratoconus. J Curr Ophthalmol 28:21–26
Correia FF, Ramos I, Lopes B et al (2012) Topometric and tomographic indices for the diagnosis of keratoconus. Int J Keratoconus Ectatic Corneal Dis 1:92–99
Shetty R, Rao H, Khamar P et al (2017) Keratoconus screening indices and their diagnostic ability to distinguish normal from ectatic corneas. Am J Ophthalmol 181:140–148
Villavicencio OF, Gilani F, Henriquez MA et al (2014) Independent population validation of the Belin/Ambrósio enhanced ectasia display: implications for keratoconus studies and screening. Int J Keratoconus Ectatic Corneal Dis 3:1–8
Brunner M, Czanner G, Vinciguerra R et al (2018) Improving precision for detecting change in the shape of the cornea in patients with keratoconus. Sci Rep 8:1–7
Greenstein SA, Fry KL, Hersh PS (2012) Effect of topographic cone location on outcomes of corneal collagen cross-linking for keratoconus and corneal ectasia. J Refract Surg 28:397–405
Santodomingo-Rubido J, Carracedo G, Suzaki A et al (2022) Keratoconus: an updated review. Contact Lens Anterior Eye 45:101559
Hashemi H, Heydarian S, Hooshmand E et al (2020) The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea 39:263
Mas Tur V, MacGregor C, Jayaswal R et al (2017) A review of keratoconus: diagnosis, pathophysiology, and genetics. Surv Ophthalmol 62:770–783
Argus WA (1995) Ocular hypertension and central corneal thickness. Ophthalmology 102:1810–1812
Congdon NG, Broman AT, Bandeen-Roche K et al (2006) Central corneal thickness and corneal hysteresis associated with glaucoma damage. Am J Ophthalmol 141:868–875
Medeiros FA, Meira-Freitas D, Lisboa R et al (2013) Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology 120:1533–1540
Sit AJ, Chen TC, Takusagawa HL et al (2023) Corneal hysteresis for the diagnosis of glaucoma and assessment of progression risk: a report by the American Academy of Ophthalmology. Ophthalmology 130(4):433–442
Mauri L, Uebe S, Sticht H et al (2016) Expanding the clinical spectrum of COL1A1 mutations in different forms of glaucoma. Orphanet J Rare Dis 11:1–12
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the design or execution of this study, to the writing of the manuscript, or both.
Corresponding author
Ethics declarations
Ethics committee approval
Approval was obtained by the CHULN/CAML ethics committee.
Consent
Informed consent was obtained from all individual participants included in the study.
Competing interests
Rafael Correia Barão is an Editorial Board Member of Graefe’s Archive for Clinical and Experimental Ophthalmology.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Correia Barão, R., Santos, M., Marques, R.E. et al. Keratoconus tomographic indices in osteogenesis imperfecta. Graefes Arch Clin Exp Ophthalmol 261, 2585–2592 (2023). https://doi.org/10.1007/s00417-023-06059-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-023-06059-4