Abstract
Objective
We conducted a systematic review to evaluate the outcome of macular hole (MH) treatment in eyes with uveitis.
Data source
We searched PubMed and Embase databases from inception through August 15, 2021.
Study selection
We included eyes with MHs secondary to uveitis that were managed medically or underwent pars plana vitrectomy (PPV). We excluded eyes with idiopathic MH and those secondary to causes other than uveitis.
Results
Of 27 articles, we identified 86 eyes with MH secondary to uveitis that received either conservative medical treatment alone or PPV with adequate follow-up. The mean (± SD) age of patients included in this review was 46.6 (± 16.8) years; 60.5% were males. The most common etiology of uveitis was Behçet’s disease (34.6%) and toxoplasmosis (19.7%). The most common anatomical location of uveitis was posterior (59.3%) followed by panuveitis (35.2%). The mean (± SD) baseline LogMAR vision was 1.1 (± 0.5). Conservative medical treatment was employed in 34.9%, while PPV was performed in 65.1% of eyes. Overall, the mean (SD) LogMAR vision improved from 1.1 (± 0.5) at baseline to 0.7 (± 0.5) after treatment. Inflammation-related MHs were closed in 40% of eyes after conservative therapy and in 87.5% of eyes after PPV. Visual improvement occurred in most eyes (83.9%) that had successful closure of their MH.
Conclusions
Visual improvement occurs in most eyes that had successful closure of their inflammation-related MH. Conservative medical control of uveitis may lead to closure of inflammation-related MHs and is an important step prior to surgery, if required. Surgical intervention for inflammation-related MHs is associated with good functional and anatomical results.

Similar content being viewed by others
Introduction
Uveitis is an inflammatory condition of the uveal tract that arises due to infectious or noninfectious disease processes and can cause devastating visual loss. The disease prevalence is relatively low at 58–115 per 100,000 people; however, uveitis is the cause of 15% of blindness among young, working-age adults in the USA, and 19% of those with uveitis are estimated to develop permanent visual loss [1, 2]. Even mild uveitis involving the anterior uvea may result in pathological changes in the retina and vitreous. Eyes that develop uveitis-related vitreous hemorrhage, cystoid macular edema (CME), macular holes (MHs), and epiretinal membranes (ERMs) are especially susceptible to irreversible visual deficits [1, 3].
Initial therapy for uveitis involving the posterior segment involves periocular corticosteroid injections as well as oral corticosteroids. Resistant inflammation is addressed with systemic immunomodulatory therapies and/or sustained intravitreal steroid therapy [4]. Medical therapy to control uveitis is an important step before surgical interference to fix MHs which was shown to be associated with MH closure in a small case series [5].
Currently, pars plana vitrectomy (PPV) is performed for both diagnostic and therapeutic indications including vitreous biopsies, removal of significant media opacity, treatment of intractable macular edema, and other structural complications such as treatment of rhegmatogenous and tractional retinal detachments [6]. The literature provides conflicting evidence regarding PPV for MHs in uveitic eyes. Assimilation of data from case reports, case series, and retrospective reviews in recent literature suggest inconsistent visual outcomes making it challenging to conclude whether the potential gain in visual acuity is worth the surgical risk and the cost of surgery [3, 7, 8]. In this review, we aim to evaluate the current literature on the outcome of conservative medical treatment and PPV in inflammation-related MHs.
Epidemiology and pathogenesis of inflammation-related macular holes
A MH is a defect in the neurosensory retina that is most commonly idiopathic but may also occur as a result of trauma or due to uveitis [9]. Inflammation-related MHs tend to affect those in the third and fourth decades of life compared with idiopathic MHs, which occur in the sixth to seventh decades of life [3, 6]. Macular hole is a rare complication of uveitis [2, 3, 10, 11]; a recent review estimated a 2.5% prevalence of MH in uveitic eyes [12].
The pathogenesis of MHs in uveitis is still unclear, but certain mechanisms have been suggested. Inflammatory involvement of the macula may cause localized tissue necrosis and degeneration of the inner retinal layers, leading to MH formation [2]. Persistent hyaloid membrane traction, chronic or recurrent CME, ERM, and vitreomacular traction (VMT) may also play a role [2, 5, 7]. The ongoing inflammation and vitritis may cause vitreal adhesion and contraction, generating another form of tractional forces on the macula [1, 3, 13]. Mizuno et al. described a case of acute inflammation in a Vogt-Koyanagi-Harada (VKH) patient causing posterior vitreous detachment and severe VMT leading to MH formation. The authors postulated that inflammation-related MH can occur when chronic inflammation causes RPE migration along the retina, eventually contracting and creating traction on the macula [13].
Methods
We conducted this review following the methodology guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews, in order to summarize findings from the heterogeneous data available regarding inflammation-related MHs [14]. The primary outcome of the review was to evaluate the visual and anatomical results of conservative and surgical treatment of inflammation-related MHs.
Search methods
We used PubMed and EMBASE scholar databases to search for the relevant studies. We used various combinations of the following search keywords: macular hole, uveitis, panuveitis, vitritis, retinitis, choroiditis, chorioretinitis, retinochoroiditis, inflammation, and vitrectomy. The latest search was conducted on August 15, 2021. We selected studies that were published in peer-reviewed journals and in English. Additional studies were included from the reference list of the eligible studies. We did not apply restrictions regarding publication status.
Inclusion and exclusion criteria
We selected studies that provided data on MH that occurred in the context of uveitis, and either underwent PPV or had uveitis control with adequate follow-up of the status of the MH following treatment. Due to the scarcity of data available, we included case series and case reports. We excluded studies on primary MHs, as well as those secondary to causes other than uveitis, and MH associated with retinal detachment. We also excluded studies that described MH as a complication of uveitis without focusing on the status and follow-up of the MH after treatment. Finally, we included additional selected papers to provide a brief narrative overview of the epidemiology, pathogenesis, and experts’ opinions on this topic.
Data extraction
We reviewed eligible studies and extracted data regarding age, sex, etiology and anatomical location of uveitis, size of MH, baseline and post-treatment visual acuity (VA), duration of uveitis control prior to performing PPV, and the status of the MH following treatment. Data extraction and filtering were performed by two independent (MS and DT) reviewers. Disagreements among the reviewers were solved through discussion to reach a consensus. We converted visual acuity values to the Logarithm of the Minimum Angle of Resolution (LogMAR) values for the purpose of analysis. We conducted a narrative synthesis of the results included in this review.
Results
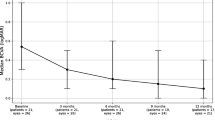
We found a total of 7631 studies that were filtered down to 27 eligible studies based on our preset eligibility criteria (Fig. 1). These included 17 case reports and 10 case series; we only included eyes that fulfilled our inclusion criteria in each case series. The latter studies were composed of 86 eyes with inflammation-related MH. The mean (± standard deviation [SD]) age of patients included in this review was 46.6 (± 16.8) years (range, 11 to 80 years); 60.5% were males. The most common etiology of uveitis was Behçet’s disease (34.6%; 28 eyes), followed by toxoplasmosis (19.7%;16 eyes), idiopathic (9.9%; 8 eyes), VKH (6.2%; 5 eyes), and viral retinitis (6.2%; 5 eyes). The remaining 23.4% of eyes had other etiologies that are listed in Tables 1 and 2.[15,16,17,18,19,20,21,22,23,24,25] The most common anatomical location of uveitis was posterior (59.3%; 32 eyes), followed by panuveitis (35.2%; 19 eyes), anterior uveitis (7.4%; 4 eyes), and intermediate uveitis (5.5%; 3 eyes). The mean (± SD) baseline LogMAR vision was 1.1 (± 0.5). The size of MH was not reported in 57% of eyes included in this review. In the remaining eyes, it was not possible to determine the mean minimal or basal diameter of MHs due to great heterogeneity in reporting different dimensions of the MH.
Conservative medical treatment alone for MH was adopted in 34.9% (n = 30) of eyes, while PPV was performed in 65.1% (n = 56) eyes. Surgical intervention was conducted after control of uveitis in nearly all eyes except a small number where inflammation was still active (Tables 1 and 2). Overall, the mean (± SD) LogMAR vision improved from 1.1 (± 0.5) at baseline to 0.7 (± 0.5) after treatment. The mean (± SD) LogMAR visual improvement after conservative and PPV treatment was 0.3 (± 0.6) and 0.5 (± 0.5), respectively. Overall, inflammation-related MHs were closed in 70.9% of eyes. Forty percent of MHs closed after conservative treatment, and 87.5% were closed after PPV. Visual improvement occurred in most eyes (83.9%) that had successful closure of their MH. Eighty percent of MHs that failed to close after treatment were secondary to Behçet’s disease. The mean (± SD) duration for MH closure after medical therapy was 4 (± 3.3) months.
Discussion
We performed a systematic review of the literature to analyze the available data on the clinical characteristics and treatment outcomes of inflammatory MHs. We extracted data from 84 eyes with inflammation-related MHs from 27 studies. We found that medical control of uveitis alone may lead to closure of inflammation-related MHs in 40% of cases and that surgical intervention is associated with anatomical closure in approximately 90% of cases, and is worth attempting.
Prior reports have demonstrated closure of inflammation-related MHs and visual recovery with conservative medical treatment alone. Ucar et al. described a case of full-thickness macular hole (FTMH) resolution with improved VA after the administration of oral corticosteroids and subcutaneous interferon-alfa2b in a patient with uveitis secondary to Behçet’s disease [26]. Additional evidence from small retrospective case series showed that control of ocular inflammation led to closure of MH and improvement in VA [5, 27, 28]. Visual improvement is often attributed to both control of inflammation and closure of the MH. Although MH closure may occur following medical treatment in 40% of cases as demonstrated in this review, chronic holes which are large in diameter may require surgical intervention [5, 9]. We found that data regarding the size of the MH was not available for most eyes that were treated medically; however, the majority of eyes that demonstrated successful closure appeared to be of a small to medium size.
The optimal surgical technique is yet to be established as treatment outcomes are often unpredictable [2, 11, 29, 30]. Despite inconsistent visual acuity outcomes with surgical intervention for inflammation-related MHs compared to idiopathic holes, the majority of eyes in this review demonstrated successful closure of the MHs following PPV with corresponding visual improvement [2, 11, 31, 32]. Lower anatomical and functional success rates compared with idiopathic MH may be due to retinal atrophy, CME, and chorioretinal ischemia that may occur secondary to chronic inflammation [3]. It is of note that, MHs that develop secondary to vitreous inflammation with subsequent vitreoretinal traction may be associated with a better chance of closure than those due to retinal ischemia and atrophy [29, 33, 34]. Perhaps, this is may be the reason that Behçet’s disease was the most frequent cause of persistent MH after treatment. Newer surgical techniques may improve the success rate of PPV for inflammation-related MHs. The “inverted flap technique” has been used for large macular holes and entails incomplete ILM peeling, keeping part of the ILM attached to the macular hole edge with a flap repositioned to cover the hole cavity [9]. Several case reports, as well as a case series, have suggested using an inverted ILM flap technique citing both improved closure rates of up to 100% and improved postoperative VA in MHs with a diameter greater than 400 µm [35]. The ILM acts as a scaffold for Muller cell growth and stimulates gliosis [9, 35]. Hirano et al. reported edge approximation in inflammation-related MH as early as 6 h postoperatively per optical coherence tomography (OCT) images, with complete closure by 6 months using ILM flaps. However, the repair did not correlate with improved VA [36]. Sen et al. reported successful closure of an inflammation-related MH with visual improvement using this surgical technique. In case the ILM flap is not attempted, it is important to make sure that ILM is completely removed from the edges of the hole to relieve all traction around the hole. Figure 2 shows successful closure of MH following PPV in an eye with posterior uveitis secondary to Behçet’s disease.
Optical coherence tomography (OCT) scans of the retina of right eye of a patient with posterior uveitis secondary to Behçet’s disease; (A) the OCT scan shows full thickness macular hole; (B) shows closure of the MH after pars plana vitrectomy with internal limiting membrane peeling and gas tamponade
In the context of uveitis, MH is commonly associated with ERM and CME. Optimal results may be linked to good pre-operative inflammatory control and is mostly encountered in eyes without severe, preexisting macular damage. To achieve the best outcomes, it is prudent to determine if the associated pathology is primarily responsible for the visual compromise. In case of coexisting CME, treatment of CME should be attempted first. If the vision remains compromised, then it is plausible to consider PPV. It is important to note that ERM in uveitic eyes may be adherent to the retina; thus, caution should be entertained when peeling these membranes. Also, many of these patients are young with absent PVD, unlike primary ERM.
Because vitrectomy has a proinflammatory effect, it is considered preferable to operate on an eye with no evidence of active inflammation, ideally for at least 3 months prior to surgery [7]. However, preoperative control of inflammation was not always achieved in all studied cases [13, 37, 38]. We find the use of intraoperative dexamethasone implant at the time of PPV surgery to be a feasible option for tightening the perioperative control of intraocular inflammation and counteract postoperative inflammation and macular thickening that may occur [39].
Lack of coherent methodology in reporting the data investigated by this review is a shortcoming identified by previous authors, but not yet addressed [6]. Most studies examining macular pathology in uveitis still involve varied uveitic etiologies and anatomic locations as well as included a small number of eyes. In addition, the impact of progressive cataract formation on long-term vision was not available except in few studies [2, 22]. There is also inconsistent reporting of pertinent details, including ocular inflammation metrics such as vitreous cells and haze scores, and OCT changes in macular structure before and after surgery. In addition, there is a risk of publication bias that applies to case reports and small series which tend to report more frequently on positive outcomes. Future studies should aim to address those shortcomings to better assess the outcome of medical and surgical management of inflammation-related MH.
Conclusions
Conservative treatment with corticosteroids and immunomodulatory therapy is widely accepted initial management as it avoids surgical risk and is necessary to control intraocular inflammation preoperatively. Although there is insufficient data on visual and anatomical outcomes of PPV for inflammation-related MH, taking the available data into account, we conclude that current literature provides some evidence to support intervention with PPV for MHs that do not resolve with pharmacologic control of uveitis.
References
Tomkins-Netzer O, Talat L, Bar A et al (2014) Long-term clinical outcome and causes of vision loss in patients with uveitis. Ophthalmology 121:2387–2392
Callaway NF, Gonzalez MA, Yonekawa Y et al (2018) Outcomes of pars plana vitrectomy for macular hole in patients with uveitis. Retina 38:S41–S48
Branson SV, McClafferty BR, Kurup SK (2017) Vitrectomy for epiretinal membranes and macular holes in uveitis patients. J Ocul Pharmacol Ther 33:298–303
Babu K, Mahendradas P (2013) Medical management of uveitis—current trends. Indian J Ophthalmol 61:277–283
Bonnin N, Cornut PL, Chaise F et al (2013) Spontaneous closure of macular holes secondary to posterior uveitis: case series and a literature review. J Ophthalmic Inflamm Infect 3(1):34. https://doi.org/10.1186/1869-5760-3-34
Becker M, Davis J (2005) Vitrectomy in the treatment of uveitis. Am J Ophthalmol 140:1096–1105
Kurup SK, Fine HF, Thomas T (2017) Whether to ignore the macular hole in a uveitic patient: a discussion of the pros and cons of elective macular surgery in a challenging population. Ophthalmic Surg Lasers Imaging Retina 48:956–960
Arana B, Fonollosa A, Artaraz J, Martinez-Berriotxoa A, Martinez-Alday N (2014) Macular hole secondary to toxoplasmic retinochoroiditis. Int Ophthalmol 34:141–143. https://doi.org/10.1007/s10792-013-9754-2
Sen P, Shah H, Geogre A (2019) Inverted flap technique for large macular hole secondary to chronic uveitis. Middle East Afr J Ophthalmol 26:43
Nussenblatt RB (1986) Macular alterations secondary to intraocular inflammatory disease. Ophthalmology 93:984–988
Woo SJ, Yu HG, Chung H (2010) Surgical outcome of vitrectomy for macular hole secondary to uveitis. Acta Ophthalmol 88:e287-288
Tingting Liu HB, Wang X, Gao Y, Wang G, Ma W (2015) Macular abnormalities in Chinese patients with uveitis. Optom Vis Sci 92:858–862
Mizuno M, Fujinami K, Watanabe K, Akiyama K (2015) Macular hole associated with Vogt-Koyanagi-Harada Disease at the acute uveitic stage. Case Rep Ophthalmol 6:328–332
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE (2018) PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/m18-0850
Karthikeya R, Ravani RD, Kakkar P et al (2017) Intravitreal cysticercosis with full thickness macular hole: management outcome and intraoperative optical coherence tomography features. BMJ Case Rep 2017:bcr2016218645. https://doi.org/10.1136/bcr-2016-218645
Buckle M, Majid MA, Lee R, Steeples LR (2017) Full-thickness macular hole: a rare complication of Borreliaburgdorferi neuroretinitis. BMJ Case Rep 2017.https://doi.org/10.1136/bcr-2016-219019
Kobayashi I, Inoue M, Okada AA, Keino H, Wakabayashi T, Hirakata A (2008) Vitreous surgery for macular hole in patients with Vogt-Koyanagi-Harada disease. Clin Experiment Ophthalmol 36:861–864. https://doi.org/10.1111/j.1442-9071.2008.01903.x
Shah S, Manayath GJ, Ranjan R, Venkatapathy N, Kanakath A (2021) Sequential multimodal imaging of isolated necrotic full-thickness macular hole secondary to toxoplasma retinochoroiditis. Am J Ophthalmol Case Rep 23:101193. https://doi.org/10.1016/j.ajoc.2021.101193
Seth A, Raina UK, Thirumalai S, Batta S, Ghosh B (2015) Full-thickness macular hole in Bartonella henselae neuroretinitis in an 11-year-old girl. Oman J Ophthalmol 8:44–46. https://doi.org/10.4103/0974-620x.149866
Doshi S, Gulati M, Pathengay A, Hegde S (2020) Spontaneous closure of macular hole in a case of toxoplasma retinochoroiditis. Indian J Ophthalmol 68:915–916. https://doi.org/10.4103/ijo.IJO_1262_19
Halkiadakis I, Pantelia E, Giannakopoulos N, Koutsandrea C, Markomichelakis NN (2003) Macular hole closure after peribulbar steroid injection. Am J Ophthalmol 136:1165–1167. https://doi.org/10.1016/s0002-9394(03)00668-8
Mesquida M, Pelegrín L, Llorenç V, Espinosa G, Ríos J, Dick AD, Adán A (2013) Pars plana vitrectomy for vitreoretinal complications of Behçet uveitis. Eur J Ophthalmol 23:119–128. https://doi.org/10.5301/ejo.5000194
Sheu SJ, Yang CA (2004) Macular hole in Behcet’s disease. Kaohsiung J Med Sci 20:558–562. https://doi.org/10.1016/s1607-551x(09)70258-x
Sousa DC, Andrade GC, Nascimento H, Maia A, Muccioli C (2021) Macular hole associated with toxoplasmosis: a surgical case series. Retin Cases Brief Rep 15:110–113. https://doi.org/10.1097/icb.0000000000000757
Park UC, Yu HG (2021) Ocular inflammation and choroidal thickness after pars plana vitrectomy in chronic recurrent stage of Vogt-Koyanagi-Harada Disease. Ocul Immunol Inflamm 29:388–395. https://doi.org/10.1080/09273948.2019.1677918
Ucar D, Atalay E, Ozyazgan Y et al (2014) An exceptional case of full-thickness macular hole closure in a patient with Behcet disease. Ocul Immunol Inflamm 22:79–81
Ebrahimi Z, Torkashvand A, Zarei M, Faghihi H, Khalili Pour E, Imani Fooldi M, Ebrahimiadib N (2021) Treatment of inflammatory macular hole: case series and review of literature. Ocul Immunol Inflamm:1-7.https://doi.org/10.1080/09273948.2020.1867871
Elhusseiny AM, Smiddy WE, Flynn HW, Schwartz SG (2019) Case series of recurring spontaneous closure of macular hole. Case Rep Ophthalmol Med 2019:2398342. https://doi.org/10.1155/2019/2398342
Al-Dhibi H, Abouammoh M, Al-Harthi E et al (2011) Macular hole in Behcet’s disease. Indian J Ophthalmol 59:359–362
Tanaka R, Obata R, Sawamura H, Ohtomo K, Kaburaki T (2014) Temporal changes in a giant macular hole formed secondary to toxoplasmic retinochoroiditis. Can J Ophthalmol 49:e115-118. https://doi.org/10.1016/j.jcjo.2014.06.006
Kelly NE, Wendel RT (1991) Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol 109:654–659. https://doi.org/10.1001/archopht.1991.01080050068031
Shukla D, Dhawan A (2010) Evolution and management of a post-uveitis macular hole. Ophthalmic Surg Lasers Imaging:1–3.https://doi.org/10.3928/15428877-20100215-47
Gregory ME, Bhatt U, Benskin S, Banerjee S (2009) Bilateral full thickness macular holes in association with serpiginous choroiditis. Ocul Immunol Inflamm 17:328–329. https://doi.org/10.3109/09273940903105128
Kusaka S, Hayashi N, Ohji M, Ikuno Y, Gomi F, Tano Y (2003) Macular hole secondary to fungal endophthalmitis. Arch Ophthalmol 121:732–733. https://doi.org/10.1001/archopht.121.5.732
Mahalingam P, Sambhav K (2013) Surgical outcomes of inverted internal limiting membrane flap technique for large macular hole. Indian J Ophthalmol 61:601–603
Hirano M, Morizane Y, Kawata T et al (2015) Case report: successful closure of a large macular hole secondary to uveitis using the inverted internal limiting membrane flap technique. BMC Ophthalmol 15:83
Shukla D, Dhawan A (2011) Pharmacotherapeutic closure of a uveitic macular hole persisting after vitrectomy. Indian J Ophthalmol 59:335–336
Wu TT, Hong MC (2009) Pars plana vitrectomy with internal limiting membrane removal for a macular hole associated with Behcet’s disease. Eye (Lond) 23:1606–1607
Kirkland KA, Uwaydat SH, Siddiqui MZ, Chancellor JR, Soliman MK, Kurup S, Sallam AB (2020) Outcome of intravitreal dexamethasone implant use in uveitic eyes undergoing pars plana vitrectomy surgery. Ocul Immunol Inflamm:1–6.https://doi.org/10.1080/09273948.2020.1726970
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The aim of this topical collection is to provide up-to-date information about macular holes (MHs), discuss novel techniques used to enhance the closure rates of MHs, and describe various approaches for management of challenging cases
Rights and permissions
About this article
Cite this article
Soliman, M.K., Tohamy, D.M., Sallam, A.B. et al. Treatment outcomes of macular holes in the setting of uveitis: a scoping review. Graefes Arch Clin Exp Ophthalmol 260, 2079–2086 (2022). https://doi.org/10.1007/s00417-022-05590-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-022-05590-0