Abstract
Purpose
To study the retinal and choroidal microvascular parameters in children with nephrotic syndrome (NS).
Methods
This is a cross-sectional study. Optical coherence tomography angiography was used to evaluate the changes of retinal and choroidal microvessels in patients with NS. Thirty NS children and 20 normal controls were included in this study. The macular vessel density (VD) of the superficial capillary plexus (SCP), deep capillary plexus (DCP), choroid capillary plexus (CCP), and foveal avascular zone (FAZ) area of the SCP and DCP was quantitatively calculated. Clinical data including serum protein, blood lipid, uric acid, urea, serum creatinine, urinary protein concentration, urinary creatinine, 24-h urine volume, 24-h urinary total protein, 24-h creatinine clearance rate, and urinary albumin to creatinine ratio were collected.
Results
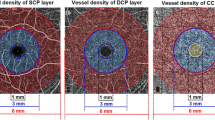
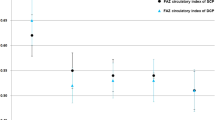
The VDs of the DCP and CCP in children with NS were significantly lower than those in controls (59.35 ± 2.45 vs. 61.15 ± 1.53, p = 0.002, 66.34 ± 1.43 vs. 67.16 ± 1.23, p = 0.042, respectively). The VD of the SCP in children with NS had a tendency to decrease compared with that in controls, but there were no significant differences. There were also no significant differences in FAZ area between the two groups. The VD of the SCP was positively correlated with serum total protein (ρ = 0.446, p = 0.014), serum albumin (ρ = 0.431, p = 0.017), and 24-h urine volume (ρ = 0.389, p = 0.034) but negatively correlated with triglyceride (ρ = − 0.450, p = 0.013), low-density lipoprotein cholesterol (ρ = −0.432, p = 0.017), urinary protein concentration (ρ = − 0.606, p < 0.001), and 24-h urinary total protein (ρ = − 0.517, p = 0.004). The VDs of the SCP, DCP, and CCP were negatively correlated with the urinary albumin to creatinine ratio (ρ = − 0.473, p = 0.008, ρ = − 0.438, p = 0.015, ρ = −0.467, p = 0.009, respectively).
Conclusions
Retinal and choroidal VDs were decreased in children with NS and paralleled the severity of kidney disease. Optical coherence tomography angiography can be used as a noninvasive method for evaluating renal injury in patients with NS.




Similar content being viewed by others
Abbreviations
- CT:
-
choroidal thickness
- CCP:
-
choroid capillary plexus
- DCP:
-
deep capillary plexus
- FAZ:
-
foveal avascular zone
- ILM:
-
inner limiting membrane
- IPL:
-
inner plexiform layer
- NS:
-
nephrotic syndrome
- OCTA:
-
optical coherence tomography angiography
- RAS:
-
renin angiotensin system
- RPE:
-
retinal pigment epithelium
- RT:
-
retinal thickness
- SCP:
-
superficial capillary plexus
- UACR:
-
urinary albumin to creatinine ratio
- VD:
-
vessel density.
References
Downie ML, Gallibois C, Parekh RS, Noone DG (2017) Nephrotic syndrome in infants and children: pathophysiology and management. Paediatr Int Child Health 37(4):248–258
Zhang W, Zhang Y, Kang L, Gu X, Wu H, Yang L (2019) Retinal and choroidal thickness in paediatric patients with hypoalbuminaemia caused by nephrotic syndrome. BMC Ophthalmol 19(1):44
De Benedetto U, Pastore MR, Battaglia Parodi M, Bandello F, Pierro L (2012) Retinal involvement in nephrotic syndrome secondary to minimal change disease. Eur J Ophthalmol 22(5):843–845
Penno G, Solini A, Zoppini G, Orsi E, Zerbini G, Trevisan R, Gruden G, Cavalot F, Laviola L, Morano S, Nicolucci A, Pugliese G, Renal Insufficiency And Cardiovascular Events (RIACE) Study Group (2012) Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care 35(11):2317–2323
Wong CW, Lamoureux EL, Cheng CY, Cheung GC, Tai ES, Wong TY, Sabanayagam C (2016) Increased burden of vision impairment and eye diseases in persons with chronic kidney disease - a population-based study. EBioMedicine 5:193–197
Wong TY, Coresh J, Klein R, Muntner P, Couper DJ, Sharrett AR, Klein BE, Heiss G, Hubbard LD, Duncan BB (2004) Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol 15(9):2469–2476
Kashani AH, Chen CL, Gahm JK, Zheng F, Richter GM, Rosenfeld PJ, Shi Y, Wang RK (2017) Optical coherence tomography angiography: a comprehensive review of current methods and clinical applications. Prog Retin Eye Res 60:66–100
Al-Sheikh M, Tepelus TC, Nazikyan T, Sadda SR (2017) Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br J Ophthalmol 101(4):449–452
Comez A, Beyoglu A, Karakucuk Y (2019) Quantitative analysis of retinal microcirculation in optical coherence tomography angiography in cases with Behcet's disease without ocular involvement. Int Ophthalmol. https://doi.org/10.1007/s10792-018-1059-z
Lahme L, Marchiori E, Panuccio G, Nelis P, Schubert F, Mihailovic N, Torsello G, Eter N, Alnawaiseh M (2018) Changes in retinal flow density measured by optical coherence tomography angiography in patients with carotid artery stenosis after carotid endarterectomy. Sci Rep 8(1):17161
Yu J, Xiao K, Huang J, Sun X, Jiang C (2017) Reduced retinal vessel density in obstructive sleep apnea syndrome patients: an optical coherence tomography angiography study. Invest Ophthalmol Vis Sci 58(9):3506–3512
Lanzillo R, Cennamo G, Criscuolo C, Carotenuto A, Velotti N, Sparnelli F, Cianflone A, Moccia M, Brescia Morra V (2018) Optical coherence tomography angiography retinal vascular network assessment in multiple sclerosis. Mult Scler 24(13):1706–1714
O'Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP (2018) Association of preclinical alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol 136(11):1242–1248
Vadala M, Castellucci M, Guarrasi G, Terrasi M, La Blasca T, Mule G (2019) Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefes Arch Clin Exp Ophthalmol 257(8):1687–1698
Shin YU, Lee DE, Kang MH, Seong M, Yi JH, Han SW, Cho H (2018) Optical coherence tomography angiography analysis of changes in the retina and the choroid after haemodialysis. Sci Rep 8(1):17184
Nephrology Group, Chinese Paediatric Society, Chinese Medical Association (2017) Evidence-based guideline for the diagnosis and treatment of steroid-sensitive, relapse/dependent nephrotic syndrome in children (2016). Chin J Pediatr 10(55):729–734
Yanik Odabas O, Demirel S, Ozmert E, Batioglu F (2018) Repeatability of automated vessel density and superficial and deep foveal avascular zone area measurements using optical coherence tomography angiography: diurnal findings. Retina 38(6):1238–1245
Li XX (2017) The basis of retinal vascular disease. In: Li XX (ed) Retinal vascular disease. Peoples’s medical publishing house, Beijing, pp 51–52
Benigni A, Cassis P, Remuzzi G (2010) Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2(7):247–257
Wilkinson-Berka JL, Agrotis A, Deliyanti D (2012) The retinal renin-angiotensin system: roles of angiotensin II and aldosterone. Peptides 36(1):142–150
Filha RDS, Pinheiro SVB, Macedo ECT, Feracin V, Vieira ELM, Miranda AS, Simoes ESAC (2019) Evidence for a role of angiotensin converting enzyme 2 in proteinuria of idiopathic nephrotic syndrome. Biosci Rep 39(1). https://doi.org/10.1042/BSR20181361
Alnawaiseh M, Ertmer C, Seidel L, Arnemann PH, Lahme L, Kampmeier TG, Rehberg SW, Heiduschka P, Eter N, Hessler M (2018) Feasibility of optical coherence tomography angiography to assess changes in retinal microcirculation in ovine haemorrhagic shock. Crit Care 22(1):138
Zhang Y, Weng H, Li Q, Wang Z (2018) Changes in retina and choroid after haemodialysis assessed using optical coherence tomography angiography. Clin Exp Optom 101(5):674–679
Huang QX, Zhu PL, Huang F, Lin F, Gao ZH, Chen FL, Huang JG (2013) The relationship between association of microalbuminuria and retinal vessel diameter in population with essential hypertension. Zhonghua Nei Ke Za Zhi 52(4):309–312
Bao S, Huang W, Liang Y, Jiang L, Wang F, Peng Y, Zhang G, Wang N (2015) Retinal vessel diameter and chronic kidney disease in rural China: a cross-sectional study. Medicine (Baltimore) 94(49):e2076
Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375(9731):2073–2081
Mule G, Castiglia A, Cusumano C, Scaduto E, Geraci G, Altieri D, Di Natale E, Cacciatore O, Cerasola G, Cottone S (2017) Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv Exp Med Biol 956:279–306
Yun L, Xu R, Zhang L, Li G, Huang S, Yao Y, Li J (2014) The role of microalbuminuria in arterial endothelial dysfunction in hypertensive patients with carotid plaques. Int Heart J 55(2):153–159
Klein R, Sharrett AR, Klein BE, Chambless LE, Cooper LS, Hubbard LD, Evans G (2000) Are retinal arteriolar abnormalities related to atherosclerosis?: the atherosclerosis risk in communities study. Arterioscler Thromb Vasc Biol 20(6):1644–1650
Wang R, Liang Z, Liu X (2019) Diagnostic accuracy of optical coherence tomography angiography for choroidal neovascularization: a systematic review and meta-analysis. BMC Ophthalmol 19(1):162
Acknowledgments
Thanks are due to Xuan Zhao, Northeast Agricultural University, for her support in data processing.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 81670841), and Beijing Natural Science Foundation (grant number 7172218). The funding body had no influence on the design, collection, analysis, or interpretation of the data or on the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Liu Yang and Wenbo Zhang designed the study. Wenbo Zhang, Lei Kang, Xiaopeng Gu, Yadi Zhang, Ruilin Zhu, Liang Zhao, Xiaosha Wang, and Hailong Wu were responsible for the data collection. Wenbo Zhang, Lei Kang, and Yadi Zhang contributed to the data analysis. Wenbo Zhang was mainly responsible for writing the manuscript. Liu Yang revised and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the parents/legal guardians of the participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Kang, L., Zhang, Y. et al. Quantitative analysis of retinal and choroidal microvascular parameters using optical coherence tomography angiography in children with nephrotic syndrome: a pilot study. Graefes Arch Clin Exp Ophthalmol 258, 289–296 (2020). https://doi.org/10.1007/s00417-019-04561-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04561-2