Abstract
Background
Presence of oligoclonal bands (OCBs) restricted to cerebrospinal fluid (CSF) characterizes most patients with multiple sclerosis (MS). Few data are available on the frequency of MS diagnosis and the main alternative diagnoses in patients with an initial central nervous system (CNS) demyelinating event and CSF IV pattern, the so-called ‘mirror pattern’.
Methods
Seventy-six patients presenting with OCBs pattern IV after a clinical attack suggestive of CNS demyelinating event were included in the study. Diagnostic work-up, including blood, CSF, and paraclinical examinations, and 2 years of clinical and radiological follow-up were evaluated.
Results
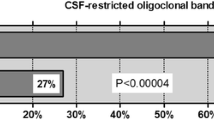
Pattern IV occurred in 15.1% of patients. Twenty-five patients (32.8%) received a diagnosis of MS, thirty-two (42.1%) an alternative diagnosis, and nineteen (25%) remained without definite diagnosis. Most frequent alternative diagnosis was encephalopathy with atypical MRI lesions of probable vascular origin (19.7%). MS was significantly more common in patients with type IV OCB pattern (25 of 76) than in a group of patients presenting with type I OCB pattern (32 of 168, p = 0.017).
Conclusion
The diagnosis of MS is common in patients who present with OCBs pattern IV. However, other CNS disorders, particularly vascular encephalopathy, should be carefully considered.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
For decades, IgG oligoclonal bands (OCBs) restricted to CSF have been recognized as a hallmark of multiple sclerosis (MS) [1]. The latest revision of the McDonald criteria incorporates OCBs in the diagnostic process for MS, allowing accurate and earlier diagnosis in patients with monophasic syndromes lacking demonstration of dissemination in time [2]. According to European Consensus guidelines, isoelectric focusing electrophoresis (IEF) is the most sensitive method to detect OCBs [3, 4]. Five IEF patterns have been described: i) type 1, the usual pattern, with the polyclonal IgG distribution in both CSF and serum; ii) type 2: OCBs present only in CSF; iii) type 3: OCBs confined to CSF with additional and identical bands in CSF and serum; iv) type 4 (‘mirror pattern’): identical OCBs in CSF and serum, no indication of local synthesis; v) type 5: monoclonal bands in CSF and serum [5].
The presence of CSF-restricted OCBs (patterns 2 and 3) has high sensitivity and specificity for a diagnosis of MS compared with other CNS inflammatory disorders, particularly in patients with a typical first demyelinating attack [6]. In contrast, the absence of CNS-restricted OCBs has been associated with misdiagnosis of MS [7, 8], even though approximately 10% of MS patients are diagnosed with ‘OCB negative’ MS [9].
Despite revisions to diagnostic criteria for MS that have demonstrated improved sensitivity and specificity, in some patients, the diagnosis of MS often remains difficult. Several paraclinical tools have shown promise to aid accurate diagnosis in such cases: these include some MRI characteristics such as the presence of the central vein sign, cortical lesions and paramagnetic rim lesions, but also OCT [7, 10,11,12,13]. By comparison, there have been few investigations concerning IEF patterns in patients in the differential diagnosis of MS. In particular, pattern IV is an uncommon outcome of IEF in patients with the first demyelinating event [14]. To our knowledge, no data are available on (i) the risk of having MS in these patients, particularly compared to other OCB patterns; (ii) alternative diagnoses in this group of patients. The aim of this study was to characterize diagnoses in patients presenting for evaluation for MS who were found to have pattern IV OCBs.
Materials and methods
Patient cohort
In this longitudinal observational study, we systematically investigated clinical and laboratory reports of patients showing OCB IV pattern at the initial diagnostic evaluation.
This study is part of a larger study that included 502 consecutive patients aimed to study the link between the CSF analysis and the MRI profile of patients evaluated after an initial demyelinating attack.
The other inclusion criteria were:
-
a)
A first clinical attack suggestive of a CNS demyelinating disorder that led to a neurological referral to the MS Center of the Azienda Ospedaliera Universitaria of Verona, Italy, between January 2017 and January 2022;
-
b)
Availability of a complete diagnostic work-up, including blood and CSF examinations, brain and spinal cord MRI, to assess the diagnosis of MS;
-
c)
At least 2 years of clinical and radiological follow-up.
Study design
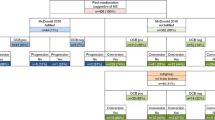
Each patient underwent a full diagnostic work-up at the time of the first demyelinating event. In case a conclusive diagnosis was not reached, patients underwent a 2-year clinical and radiological follow-up. Both at the end of the first diagnostic work-up and after 2 years of follow-up, all recruited patients were classified into three groups according to diagnosis: (i) MS according to the 2017 McDonald criteria [2]; (ii) non-MS (indication of an alternative diagnosis was required); (iii) ongoing diagnostic evaluation: conclusive diagnosis could not be made and further follow-up was required. The flowchart of the study is summarized in Fig. 1.
Each alternative diagnosis was based on the most recent international guidelines where applicable (i.e., migraine, Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease-MOGAD, neuromyelitis optica spectrum disorder [NMOSD], Sjogren syndrome, systemic lupus erythematosus) or clinical judgment where established diagnostic guidelines are lacking.
Clinical evaluation
All patients underwent clinical evaluations every 6 months, with additional visits in the case of new symptoms for relapse assessment. The site of clinical onset was recorded for each patient. Disability was assessed using the Expanded Disability Status Scale (EDSS) score [15]. A relapse was defined as a worsening of neurological impairment or the appearance of a new symptom or abnormality attributable to MS, lasting at least 24 h, in the absence of fever, and preceded by stability of at least 1 month [16]. The appearance of new or enlarging T2 lesions or gadolinium-enhancing lesions defined MRI activity. Finally, the occurrence of comorbidities was assessed.
Evaluation of OCBs and other paraclinical assessments
OCB pattern [3] was evaluated by isoelectric focusing and agarose gel immunofixation [17].
The minimum blood laboratory evaluation required at the initial work-up included a complete blood count, AST, ALT, gGT, renal profile, ANA, ENA, antiphospholipid antibodies, antithyroid antibodies, AQP4- IgG and MOG-IgG, homocysteine, vitamin B12, folate, ESR, CRP, urine examination. Infections, including HIV and syphilis, were excluded by blood analysis.
All patients underwent brain MRI with gadolinium. The size, morphology, and location of each MRI lesion were evaluated. Oval, asymmetric white matter lesions perpendicular to the ventricles (Dawson’s fingers) or lesions in the periventricular, juxtacortical, infratentorial, or spinal cord region, with a diameter > 3 mm, were considered typical of MS according to the practical guidelines for evaluating lesions on MRI [18,19,20]. We also assessed the number of contrast-enhancing lesions and fulfillment of dissemination in the space.
Visual evoked potentials (VEP) were performed in a subgroup of patients following the recommendations of the International Society for Clinical Electrophysiology of Vision [21], and latency and morphology were recorded.
Statistical analysis
Differences between groups were initially assessed using the Mann–Whitney and Chi-square/Fisher exact tests when appropriate. The χ2 test was applied to test for the effect of MRI lesion type, VEP abnormalities, spinal cord lesions, and gadolinium-enhancing lesions in identifying MS patients versus patients who did not receive a diagnosis of MS. Finally, χ2 test was used to test the difference in the frequency of MS between a comparison cohort of patients with OCB pattern I and patients without OCBs IV pattern.
Ethics approval
The local Ethics Committee approved the study, and all patients signed an informed consent (MSBioB, 2413CESC).
Data availability statement
Deidentified data will be shared upon request by qualified researchers.
Results
Among the 502 patients evaluated, 76 patients (15.1%) with the OCB IV pattern met the inclusion criteria (Fig. 1).
Fifteen (19.7%) received a diagnosis of RRMS immediately after the initial diagnostic work-up (Table 1), while ten received an alternative diagnosis (Fig. 2, Table 2). In 51 cases, inconclusive diagnosis required further clinical and radiological follow-up.
Atypical MRI lesions in patients with an alternative diagnosis. a Bilateral small juxtacortical frontal T2-hyperintese lesions, the larger one within the right inferior frontal gyrus (5 mm longitudinal axis) in a patient with anti-MOG encephalopathy. b Bilateral small juxtacortical, deep and periventricular frontal parietal and T2-hyperintese lesions, partially confluent within the posterior white matter in a patient with vascular encephalopathy
Among the 51 patients with an ongoing diagnostic investigation at the initial diagnostic evaluation, 10 received a diagnosis of MS, 14 of encephalopathy with atypical MRI lesions of probable vascular origin and 8 received an alternative diagnosis (Table 2).
MS patients were younger (mean age 36.8 ± 13.8 years) than patients with an alternative diagnosis (mean age 46.2 ± 12.6 years, p = 0.015) and showed greater disability (median EDSS 2.5 [0–7] vs 1 [0–6.5], p = 0.029). Diagnoses at initial work-up and after 2 years are shown in Table 2. Table 3 details how patients fulfilled the diagnostic criteria for MS.
In the MS group with ‘mirror pattern’, 37.5% of patients had abnormal visual evoked potentials, without a previously reported optic neuritis. Spinal cord lesions were detected in 68.4% of cases, and gadolinium enhancement was present in 58.8%. Of these, only 8% had atypical MRI lesions. In contrast, atypical MRI lesions were common in the ‘non-MS’ group (23/51, 45.1%, p = 0.002, Table 4).
Comorbidities were noted in patients with type IV OCB. Comorbidities are shown in Table 5. The most common comorbidity was thyroid pathology (n = 9, 11.8%) and rheumatic signs or symptoms were also common (n = 9, 11.8%).
Among the 502 patients, 168 showed type I OCB. After a 2-year clinical follow-up, 32 (19.0%) received a final MS diagnosis (mean age 37.4 ± 11.4 years), while 136 received an alternative diagnosis (mean age 44.1 ± 10.3 years, p = 0.003). Encephalopathy with atypical CNS lesions of probable vascular origin (patients with small vessels disease who developed small vascular lesions atypical for MS) was the most common diagnosis (31, 18.4%). Notably, the diagnosis of MS was significantly more common in patients with a ‘mirror pattern’ (25/76) than in patients with type I OCB pattern (32/168, 19.0%, p = 0.017).
Finally, 258 patients presenting an initial demyelinating attack and with the presence of OCB (pattern 2 or 3) were also evaluated. Among these, 221 (85.6%) received the diagnosis of MS, while the remaining 37 are still under investigation with a diagnosis of encephalopathy with atypical CNS lesions of probable vascular origin being the most frequent diagnosis.
Discussion
Patients with MS without CSF type II and III pattern represent approximately 10%–12% of cases [9, 14]. These cases have been associated with suspected misdiagnosis of MS [8], and OCB patterns in patients without type II and III pattern are infrequently reported in the literature.
In our cohort, in patients with type I and type IV OCB patterns, MS was diagnosed in approximately 1 in 5 and 1 in 3 patients, respectively. This observational, real-life longitudinal study, based on clinical practice, provided evidence that the occurrence of MS in those patients with a ‘mirror pattern’ is not uncommon. Furthermore, patients who experienced a first possible demyelinating event and had a IV OCB pattern showed an increased risk of MS compared to type I OCBs. Encephalopathy with atypical lesions of probable vascular origin was the most common alternative diagnosis in patients with both type IV and I patterns, supporting data from larger cohorts of patients presenting for suspected MS [7].
MS patients with a ‘mirror pattern’ were characterized by a younger age than the group without MS suggesting perhaps that advanced age is a potential ‘red flag’ in patients presenting with type IV OCBS and evaluation for MS. While type of clinical onset, other than optic neuritis, was not useful in discriminating patients with or without MS in the “mirror pattern” group, atypical MRI lesions smaller than 3 mm in their long axis and located in specific brain regions [19] were more frequent in patients with alternative diagnoses. The presence of gadolinium-enhancing lesions or spinal cord lesions was not a discriminant for the diagnosis of MS. Among paraclinical tests, VEP alterations were not different between patients who ultimately received a diagnosis of MS or not. This was partially unexpected, as abnormal VEPs are typical of MS and useful in the diagnostic process [7]. Nevertheless, the relatively low number of patients with available VEP data may have influenced our results. Ongoing research has focused on additional characteristics of MS lesions, such as the “central vein sign” [22] or the presence of a paramagnetic rim [23], but data in patients with MS and type IV OCBS are unfortunately lacking. Finally, according to the diagnoses that have been obtained in patients with IV OCB pattern, we suggest the application of an extended panel of blood examinations, with a particular focus on the exclusion of hematological and rheumatological morbidities.
There is consistent evidence that the presence of OCBs limited to the CNS is associated with increased disability. OCB + patients had a higher risk of achieving an EDSS of 6 and early disability progression [24, 25]. Furthermore, increased cortical lesion burden and increased expression of inflammatory markers have been associated with CSF-restricted oligoclonal bands in MS [26]. Unfortunately, these data are completely lacking in MS and ‘mirror pattern’ patients, thus suggesting that a more accurate CSF profiling, including proteomic analysis, of ‘mirror pattern’ patients would help in the diagnostic work-up and characterization of the risk of disability progression in these patients. Interestingly, increased disability at the initial presentation was noted in patients diagnosed with MS after 2 years of follow-up with type IV OCBS compared to patients with alternative diagnoses.
The study has a number of limitations. These include referral bias, the relatively small number of patients, and the short duration of follow-up. Furthermore, CSF analysis was not repeated, thus potentially underestimating the occurrence of OCB restriction when repeating the analysis in patients with early MS [27]. Few data are available from longitudinal studies with repeated lumbar punctures. In a cohort study, 12.5% of patients who underwent a second lumbar puncture, had changes in OCB status, with in most cases disappearance of OCBs, without a significant impact on the final diagnosis [28]. Furthermore, a repeated lumbar puncture could provide value in evaluating treatment effect on the intrathecal compartment, with in some cases in disappearance of OCBs after treatment for MS [29, 30]. We believe that consideration should be given to repeating the lumbar puncture and CSF analysis if clinical suspicion is high but results of CSF are equivocal, negative, or show only a single band [27]. Comparison of the clinical characteristics of patients with MS and type IV and type I OCB patterns to patients with MS with CSF-restricted OCBs may have been informative, but was beyond the scope of this study. Finally, the presence of a ‘mirror pattern’ cannot exclude the presence of intrathecal IgG synthesis. Accordingly, additional methods that could increase sensitivity for intrathecal humoral activity should be validated in clinical practice [31,32,33].
In conclusion, although pattern IV is not the most common OCB pattern in MS, in our cohort, MS was frequently diagnosed in patients with this OCB pattern. Moreover, the risk of MS was higher in patients presenting for MS evaluation with OCB pattern IV compared to pattern I, perhaps suggesting that MS in that such patients warrant careful consideration for MS while ruling out other inflammatory CNS disorders. Future studies in larger cohorts are needed to confirm these data to inform diagnostic evaluation and to clarify the natural history of MS in patients without signs of intrathecal IgG synthesis.
Data availability
Deidentified data will be shared on request from a qualified investigator.
References
Kabat EA, Freedman DA et al (1950) A study of the crystalline albumin, gamma globulin and total protein in the cerebrospinal fluid of 100 cases of multiple sclerosis and in other diseases. Am J Med Sci 219(1):55–64
Thompson AJ et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17(2):162–173
Andersson M et al (1994) Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry 57(8):897–902
Bjellqvist B et al (1982) Isoelectric focusing in immobilized pH gradients: principle, methodology and some applications. J Biochem Biophys Methods 6(4):317–339
Jin H et al (2023) Application of oligoclonal bands and other cerebrospinal fluid variables in multiple sclerosis and other neuroim diseases: a narrative review. Ann Transl Med 11(7):282
Petzold A (2013) Intrathecal oligoclonal IgG synthesis in multiple sclerosis. J Neuroimmunol 262(1–2):1–10
Calabrese M et al (2019) “Better explanations” in multiple sclerosis diagnostic workup: A 3-year longitudinal study. Neurology 92(22):e2527–e2537
Katsarogiannis E et al (2023) Absence of oligoclonal bands in multiple sclerosis: a call for differential diagnosis. J Clin Med 12(14):4656
Dobson R et al (2013) Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 84(8):909–914
Borrelli S et al (2024) Central vein sign, cortical lesions, and paramagnetic rim lesions for the diagnostic and prognostic workup of multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 11(4):e200253
Cagol A et al (2024) Diagnostic performance of cortical lesions and the central vein sign in multiple sclerosis. JAMA Neurol 81(2):143–153
Kenney RC et al (2022) The role of optical coherence tomography criteria and machine learning in multiple sclerosis and optic neuritis diagnosis. Neurology 99(11):e1100–e1112
Solomon AJ, Naismith RT, Cross AH (2019) Misdiagnosis of multiple sclerosis: Impact of the 2017 McDonald criteria on clinical practice. Neurology 92(1):26–33
Franciotta D et al (2008) Oligoclonal IgG band patterns in inflammatory demyelinating human and mouse diseases. J Neuroimmunol 200(1–2):125–128
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33(11):1444–1452
Poser CM et al (1983) New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol 13(3):227–231
Gastaldi M et al (2017) Cerebrospinal fluid analysis and the determination of oligoclonal bands. Neurol Sci 38(Suppl 2):217–224
Barkhof F et al (1997) Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain 120(Pt 11):2059–2069
Filippi M et al (2019) Assessment of lesions on magnetic resonance imaging in multiple sclerosis: practical guidelines. Brain 142(7):1858–1875
Filippi M et al (2013) Gray matter damage predicts the accumulation of disability 13 years later in MS. Neurology 81(20):1759–1767
Odom JV et al (2016) ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol 133(1):1–9
Maggi P et al (2018) Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol 83(2):283–294
Absinta M, Sati P, Reich DS (2016) Advanced MRI and staging of multiple sclerosis lesions. Nat Rev Neurol 12(6):358–368
Ben Noon G et al (2021) Reinforcing the evidence of oligoclonal bands as a prognostic factor in patients with Multiple sclerosis. Mult Scler Relat Disord 56:103220
Rojas JI et al (2012) Oligoclonal bands in multiple sclerosis patients: worse prognosis? Neurol Res 34(9):889–892
Farina G et al (2017) Increased cortical lesion load and intrathecal inflammation is associated with oligoclonal bands in multiple sclerosis patients: a combined CSF and MRI study. J Neuroinflammation 14(1):40
Freedman MS et al (2005) Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 62(6):865–870
Mermelstein M et al (2022) Repeated lumbar puncture in search of oligoclonal bands - What is the yield? J Neurol Sci 439:120298
Rejdak K, Stelmasiak Z, Grieb P (2019) Cladribine induces long lasting oligoclonal bands disappearance in relapsing multiple sclerosis patients: 10-year observational study. Mult Scler Relat Disord 27:117–120
von Glehn F et al (2012) Disappearance of cerebrospinal fluid oligoclonal bands after natalizumab treatment of multiple sclerosis patients. Mult Scler 18(7):1038–1041
Hegen H et al (2023) Cerebrospinal fluid kappa free light chains for the diagnosis of multiple sclerosis: a systematic review and meta-analysis. Mult Scler 29(2):169–181
Monreal E et al (2023) Establishing the best combination of the kappa free light chain index and oligoclonal bands for an accurate diagnosis of multiple sclerosis. Front Immunol 14:1288169
Schwenkenbecher P et al (2018) The persisting significance of oligoclonal bands in the Dawning Era of Kappa free light chains for the diagnosis of multiple sclerosis. Int J Mol Sci 19(12):3796
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. DM received honoraria for research or speaking and funds for travel from Biogen Idec, Roche, Sanofi-Genzyme, Novartis, Merck-Serono and was supported by the GR-2021-12373041 grant from Italian Ministry of Health. MC received honoraria for research or speaking and funds for travel from Roche, Sanofi-Genzyme, Merck-Serono, Biogen Idec, Teva and Novartis and was supported by the GR-2013–02-355322 grant from Italian Ministry of Health. All other authors report no conflicts of interest related to this study. #NEXTGENERATIONEU (NGEU) and funded by the Ministry of University and Research (MUR), National Recovery and Resilience Plan (NRRP), project MNESYS (PE0000006)—A Multiscale integrated approach to the study of the nervous system in health and disease (DN. 1553 11.10.2022).
Author information
Authors and Affiliations
Contributions
MC, DM, AJS contributed to the conception and design of the study.
DM, MS, AS, FV, ET, DA, SZ, VC, AT, MM, GL, BB, AJS, MC contributed to the acquisition and analysis of data, drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The local Ethics Committee approved the study, and all patients signed an informed consent (MSBioB, 2413CESC).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marastoni, D., Sicchieri, M., Pizzini, F.B. et al. Multiple sclerosis diagnosis and its differential diagnosis in patients presenting with type four ‘mirror pattern’ CSF oligoclonal bands. J Neurol 272, 207 (2025). https://doi.org/10.1007/s00415-025-12947-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-025-12947-y