Abstract
Background
New diagnostic criteria of Progressive Supranuclear Palsy (PSP) have highlighted the interest of Eye Movement Records (EMR) at the early stage of the disease.
Objectives
To investigate the metabolic brain correlates of ocular motor dysfunction using [18F] Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) in early PSP.
Methods
Retrospective observational descriptive study on longitudinal data with patients who underwent EMR and FDG-PET at the stage of suggestive and possible PSP according to Movement Disorders Society criteria. Longitudinal follow-up enables to confirm diagnosis of probable PSP. Using the Statistical Parametric Mapping software, we performed whole-brain voxel-based correlations between oculomotor variables and FDG-PET metabolism.
Results
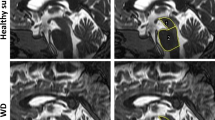
Thirty-seven patients with early PSP who fulfilled criteria of probable PSP during the follow-up were included. Decrease in the gain of vertical saccades correlated with reduced metabolism in Superior Colliculi (SC). We also found a positive correlation between mean velocity of horizontal saccades and SC metabolism as well as dorsal nuclei in the pons. Finally, increase in horizontal saccades latencies correlated with decrease of posterior parietal metabolism.
Conclusions
These findings suggest the early involvement of SC in saccadic dysfunction in the course of PSP.



Similar content being viewed by others
Data availability
Anonymized data can be shared by request from any qualified investigator as long as data transfer is in agreement with EU legislation.
References
Litvan I, Agid Y, Calne D et al (1996) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP International Workshop*. Neurology 47:1–9. https://doi.org/10.1212/WNL.47.1.1
Steele JC, Richardson JC, Olszewski J (1964) Progressive supranuclear palsy. A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy. Nuchal dystonia and dementia. Arch Neurol 10:333–359. https://doi.org/10.1001/archneur.1964.00460160003001
Respondek G, Kurz C, Arzberger T et al (2017) Which ante mortem clinical features predict progressive supranuclear palsy pathology? Mov Disord 32:995–1005. https://doi.org/10.1002/mds.27034
Respondek G, Stamelou M, Kurz C et al (2014) The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord 29:1758–1766. https://doi.org/10.1002/mds.26054
Marx S, Respondek G, Stamelou M et al (2012) Validation of mobile eye-tracking as novel and efficient means for differentiating progressive supranuclear palsy from Parkinson’s disease. Front Behav Neurosci. https://doi.org/10.3389/fnbeh.2012.00088
Shaikh AG, Factor SA, Juncos JL (2017) Saccades in progressive supranuclear palsy-maladapted, irregular, curved, and slow. Mov Disord Clin Pract 4:671–681. https://doi.org/10.1002/mdc3.12491
Rivaud-Pechoux S, Vidailhet M, Gallouedec G et al (2000) Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology 54:1029–1032. https://doi.org/10.1212/WNL.54.5.1029
Höglinger GU, Respondek G, Stamelou M et al (2017) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria: MDS Clinical Diagnostic Criteria for PSP. Mov Disord 32:853–864. https://doi.org/10.1002/mds.26987
Garbutt S (2012) Saccade abnormalities in autopsy-confirmed frontotemporal lobar degeneration and alzheimer disease. Arch Neurol 69:509. https://doi.org/10.1001/archneurol.2011.1021
Groschel K, Kastrup A, Litvan I et al (2006) Penguins and hummingbirds: midbrain atrophy in progressive supranuclear palsy. Neurology 66:949–950. https://doi.org/10.1212/01.wnl.0000203342.77115.bf
Amtage F, Maurer C, Hellwig S et al (2014) Functional correlates of vertical gaze palsy and other ocular motor deficits in PSP: an FDG-PET study. Parkinsonism Relat Disord 20:898–906. https://doi.org/10.1016/j.parkreldis.2014.05.013
Verger A, Grimaldi S, Ribeiro M et al (2021) Single photon emission computed tomography/positron emission tomography molecular imaging for parkinsonism: a fast-developing field. Ann Neurol 90:711–719. https://doi.org/10.1002/ana.26187
Grimm M-J, Respondek G, Stamelou M et al (2019) How to apply the movement disorder society criteria for diagnosis of progressive supranuclear palsy. Mov Disord 34:1228–1232. https://doi.org/10.1002/mds.27666
Buch KA, Bouffard MA, Kardon RH et al (2022) Clinical correlation between vertical gaze palsy and midbrain volume in progressive supranuclear palsy. J Neuroophthalmol 42:246–250. https://doi.org/10.1097/WNO.0000000000001393
Jaillard A, Petyt G, Morelle M (2016) [18F]-Fdg pet identified superior colliculi hypometabolism in progressive supranuclear palsy. J Alzheimers Dis Parkinsonism. https://doi.org/10.4172/2161-0460.1000278
Bhidayasiri R, Riley DE, Somers JT et al (2001) Pathophysiology of slow vertical saccades in progressive supranuclear palsy. Neurology 57:2070–2077. https://doi.org/10.1212/wnl.57.11.2070
Soetedjo R, Kaneko CRS, Fuchs AF (2002) Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol 87:679–695. https://doi.org/10.1152/jn.00886.2000
Hanes DP, Smith MK, Optican LM et al (2005) Recovery of saccadic dysmetria following localized lesions in monkey superior colliculus. Exp Brain Res 160:312–325. https://doi.org/10.1007/s00221-004-2013-z
Terao Y, Fukuda H, Ugawa Y et al (2013) New perspectives on the pathophysiology of Parkinson’s disease as assessed by saccade performance: a clinical review. Clin Neurophysiol 124:1491–1506. https://doi.org/10.1016/j.clinph.2013.01.021
Blekher T, Weaver M, Rupp J et al (2009) Multiple step pattern as a biomarker in Parkinson disease. Parkinsonism Relat Disord 15:506–510. https://doi.org/10.1016/j.parkreldis.2009.01.002
Horn AKE (2006) The reticular formation. Prog Brain Res 151:127–155. https://doi.org/10.1016/S0079-6123(05)51005-7
Büttner-Ennever JA, Horn AK, Henn V et al (1999) Projections from the superior colliculus motor map to omnipause neurons in monkey. J Comp Neurol 413:55–67. https://doi.org/10.1002/(sici)1096-9861(19991011)413:1%3c55::aid-cne3%3e3.0.co;2-k
Stanton GB, Goldberg ME, Bruce CJ (1988) Frontal eye field efferents in the macaque monkey: II. Topography of terminal fields in midbrain and pons. J Comp Neurol 271:493–506. https://doi.org/10.1002/cne.902710403
Shinoda Y, Sugiuchi Y, Takahashi M et al (2011) Neural substrate for suppression of omnipause neurons at the onset of saccades. Ann N Y Acad Sci 1233:100–106. https://doi.org/10.1111/j.1749-6632.2011.06171.x
Sparks D, Rohrer WH, Zhang Y (2000) The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vis Res 40:2763–2777. https://doi.org/10.1016/s0042-6989(00)00133-4
Bisley JW, Goldberg ME (2010) Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33:1–21. https://doi.org/10.1146/annurev-neuro-060909-152823
Pierrot-Deseilligny C, Rosa A, Masmoudi K et al (1991) Saccade deficits after a unilateral lesion affecting the superior colliculus. J Neurol Neurosurg Psychiatry 54:1106–1109. https://doi.org/10.1136/jnnp.54.12.1106
Ghika J, Tennis M, Growdon J et al (1995) Environment-driven responses in progressive supranuclear palsy. J Neurol Sci 130:104–111. https://doi.org/10.1016/0022-510X(95)00015-T
Shires J, Joshi S, Basso MA (2010) Shedding new light on the role of the basal ganglia-superior colliculus pathway in eye movements. Curr Opin Neurobiol 20:717–725. https://doi.org/10.1016/j.conb.2010.08.008
Jamadar SD, Fielding J, Egan GF (2013) Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front Psychol. https://doi.org/10.3389/fpsyg.2013.00749
Kang SS, Dionisio DP, Sponheim SR (2011) Abnormal mechanisms of antisaccade generation in schizophrenia patients and unaffected biological relatives of schizophrenia patients. Psychophysiology 48:350–361. https://doi.org/10.1111/j.1469-8986.2010.01074.x
Author information
Authors and Affiliations
Contributions
GP: reviewing and editing. JL: conceptualization, data curation, formal analysis, writing original draft, visualization. EG: conceptualization, data curation, formal analysis, writing original draft, supervision. OF: reviewing and editing. SG: reviewing and editing. JPA: reviewing and editing. MC: reviewing and editing. LK: conceptualization, data curation, formal analysis, writing original draft, visualization, supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest. All authors have approved the final article.
Ethical standard statement
Our study was approved by a local expert ethical comity for research study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pin, G., Labouré, J., Guedj, E. et al. Brain FDG-PET correlates of saccadic disorders in early PSP. J Neurol 270, 4841–4850 (2023). https://doi.org/10.1007/s00415-023-11824-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11824-w