Abstract
Background
Spinocerebellar ataxia type 8 (SCA8) is a rare autosomal dominant neurodegenerative disease caused by CTA/CTG repeat expansion in the ATXN8/ATXN8OS gene.
Methods
To analyze the frequency and clinical characteristics of SCA8 patients in mainland China, we combined polymerase chain reaction (PCR) and triplet repeat-primed PCR (TRP-PCR) to detect the CTA/CTG expansion. We studied a cohort of 362 ataxia patients in which the other known causative genes had been previously excluded, from among 1294 index patients. Positive samples were validated by southern blotting.
Results
The CTA/CTG expansion was observed in six probands, accounting for approximately 0.46% (6/1294) in all patients, and 1.66% (6/362) in patients without definite molecular diagnosis. Clinically, aside from the typical SCA8 phenotype, some patients carrying the CTA/CTG expansion exhibited the cerebellar form of multisystem atrophy (MSA-C) and ataxia with paroxysmal kinesigenic dyskinesia (PKD).
Conclusion
For the first time, we described the PKD phenotype in association with CTA/CTG expansion, suggesting that CTA/CTG expansion might play a role in the pathogenesis of paroxysmal dyskinesia symptoms.
Similar content being viewed by others
Introduction
Spinocerebellar ataxias (SCAs) are a heterogeneous group of degenerative disorders that are clinically classified as either pure, with predominant truncal and limb ataxia, or complex, complicated with additional neurological features including pyramidal, extrapyramidal, and cognitive impairment; they are inherited in an autosomal dominant or sporadic pattern [1]. To date, genetic defects have been identified in over 38 genes, with more than 45 loci implicated in total (https://neuromuscular.wustl.edu/). SCA type 8 (SCA8) is a late-onset and slowly progressive subtype with high clinical and genetic heterogeneity [2]. The typical features of SCA8 most frequently present as an adult-onset disease characterized by limbal and gait ataxia, dysarthria, and nystagmus [3, 4], but atypical phenotypes including infantile- and juvenile-onset patients and patients with cognitive or psychiatric dysfunctions and multisystem atrophy (MSA) have also been reported previously [5,6,7,8].
SCA8 is inherited in an autosomal dominant pattern with incomplete penetrance, caused by CTA/CTG trinucleotide repeat expansion in the ATXN8/ATXN8OS gene [2]. To date, most studies have reported a pathogenic repeat length of more than 80 [9]. Nevertheless, large repeats of over 800 have been reported both in clinically affected and unaffected individuals [10, 11]. The CTA/CTG repeat expansion is bi-directionally expressed as two molecules with expanded motifs. SCA8 is the first reported SCA subtype potentially associated with both an RNA gain of function caused by CUG expansion in transcripts and the pathogenic effects of polyglutamine expansion in the protein [12].
The frequency of SCA8 differs among populations and regions. The highest frequency of 5.9% was observed in Finland [13]. In addition, SCA8 has also been reported in the USA [14], Scotland [7], Mexico [14], Portugal [15], Italy [16], Brazil [17], Yugoslavia [18], Czech Republic [19], and Asian countries [20, 21] with relatively lower frequencies, indicating that SCA8 is a relatively rare SCA subtype worldwide. In Taiwan, the CTA/CTG expansion was not found to be associated with ataxia symptoms [22], but was observed in typical and atypical Parkinson’s disease cases [23]. Recently, several cases of SCA8 were reported in mainland China and Japan [20, 21].
Therefore, to further elucidate the frequency and clinical features of SCA8 in mainland China, CTA/CTG expansion was detected in a large cohort of ataxia patients.
Materials and methods
Subjects
A large cohort of 1294 unrelated Chinese SCA patients was referred to the outpatient neurology clinic of Xiangya Hospital, Central South University from February 1994 to December 2017. The clinical diagnosis of SCA was made based on the criteria proposed by Harding [24]. We recruited probands with either sporadic or autosomal dominant inheritance, excluding those with autosomal recessive inheritance. Those with known causative genetic mutations of SCA1, SCA2, SCA3, SCA6, SCA7, SCA12, SCA14, SCA17, SCA21, SCA35, SCA36, SCA40, and DRPLA subtypes were excluded in the first step. Ultimately, a selected cohort of 362 ataxia patients (121 dominantly inherited and 241 sporadic cases) and 261 healthy controls were available for analyzing the distribution of alleles at the ATXN8/ATXN8OS locus. The mean age at onset was 39.72 ± 14.44 years (range: 10–70 years old). According to our procedure, relatives of the identified patients with pathogenic repeats were recruited in a follow-up visit. Thus, another 11 clinically unaffected subjects were also included in the CTA/CTG repeat analysis (Supplementary Table 1).
Written informed consent was obtained from all subjects, as approved by the Ethics Committee and the Expert Committee of Xiangya Hospital, Central South University (equivalent to an Institutional Review Board). Genomic DNA was extracted from peripheral blood using a standard extraction method.
Standard PCR, triplet repeat-primed (TRP)-PCR, and southern blotting
We used standard PCR to detect CTA/CTG trinucleotide repeats. The target sequence covering the CTA/CTG repeat was amplified with a pair of fluorescently labeled primers designed according to published primer sequences [2, 25]. Homozygous samples were reanalyzed with fluorescent TRP-PCR to detect whether there was long pathogenic CTA/CTG repeat expansion. The primer sequences are listed in Supplementary Table 2. The fluorescent PCR product was analyzed using an ABI-Prism 3730 Genetic Analyzer, and the data were examined using Gene Mapper software (Applied Biosystems, Foster City, CA, USA). A typical sawtooth pattern indicated the expansion of the CTA/CTG trinucleotide repeats, which will be validated in southern blotting as previously described [10].
Whole exome sequencing
One patient (F6: III-1) who presented with ataxia and paroxysmal kinesigenic dyskinesia (PKD) was found to carry the CTA/CTG expansion. Whole exome sequencing (WES) was performed to exclude the PRRT2 and other paroxysmal disease pathogenic mutations using a method described previously [26].
Clinical investigation
Clinical history was acquired from all patients, and systematic clinical examination and evaluation were performed in all patients by at least two experienced neurologists. A full battery of clinical neurological and neuropsychological tests was conducted, including brain magnetic resonance imaging (MRI), electromyogram (EMG), somatosensory evoked potential (SEP), Scale for Assessment and Rating of Ataxia (SARA), International Cooperative Ataxia Rating Scale (ICARS), minimum mental state examination (MMSE), montreal cognitive assessment (MoCA), Hamilton Anxiety Scale (HAMA), Hamilton Depression Scale (HAMD), and neuropsychiatric inventory (NPI).
Data analysis
Statistical analysis was performed using the data analysis software SPSS 25.0. Proportions of ataxia patients and controls with the CTA/CTG expansion were compared using Fisher’s exact test. The threshold of statistical significance was set at p < 0.05.
Results
Genetic characteristics of CTA/CTG expansion in mainland China
Among the 362 ataxia patients in which known causative genetic mutations were excluded, a total of 300 heterozygous samples and 62 homozygous samples were revealed via standard PCR. Then, based on the TRP-PCR and Southern blotting results, six patients with CTA/CTG expansions were discovered from among the homozygous samples (Fig. 1a, b). The overall frequency of patients with CTA/CTG expansions within our cohort was 0.46% (6/1294), with the pathogenic repeat number ranging from 86 to 737 (86, 144, 171, 469, 668, and 737, Fig. 1b). The frequency in patients without definite molecular diagnosis was 1.66% (6/362). In the 261 healthy controls, expansions with more than 80 repeats were not observed (Fisher’s test, p = 0.043). In follow-up visits of first-degree relatives, we found other two individuals with CTA/CTG expansion: in F1, the father (149 repeats) of the proband, and in F5, the mother (834 repeats) of the proband. The CTA/CTG expansion was not detected in other family members.
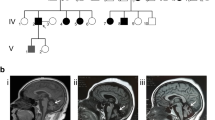
Clinical and genetic characteristics of patients with CTA/CTG expansion in the ATXN8/ATXN8OS gene. a Results of standard PCR and TRP-PCR. x-axis: product size in bp; y-axis: peak height. Panel A: two normal-sized alleles by standard PCR; panels B and C: one normal homozygous control detected by standard PCR (B) and TRP-PCR (C); panels D and E: one patient detected by standard PCR (D) (the second allele was too large to be detected), and TRP-PCR (E). b Result of southern blotting. From left to right, M = DNA molecular weight marker; 1 = F1:I-1; 2 = F1:II-1; 3 = F5:II-3; 4 = F5:III-1; 5 = F2:II-1; 6 = F3:III-1; 7 = F4:II-1; 8 = F6:III-1; 9 = normal control; c SCA8 pedigree. Squares: males; circles: females; arrow: proband; filled symbols: affected; symbols with a dot: unaffected gene mutation carriers; “+/-”: heterozygous CTA/CTG expansion; “-/-”: no CTA/CTG expansion. Due to ethical considerations, the pedigree structure has been simplified. d Brain MRI of patient F2: II-1. A: sagittal T1-weighted, showing marked cerebellar atrophy; B: axial T2-weighted, showing severe cerebellar atrophy, marked pontine atrophy, and ‘hot cross bun’ sign
Clinical characteristics of patients with CTA/CTG expansion in mainland China
In the six probands with CTA/CTG expansion, aside from the typical SCA8 phenotype, one patient showed a probable cerebellar form of multisystem atrophy (MSA-C), and one showed ataxia with a PKD phenotype. The clinical features of these patients are detailed in Table 1. The six probands had no evident family history, although the first-degree relatives of two probands (F1: II-1 and F5: III-1) were gene mutation carriers (Fig. 1c). The age at onset for the six patients ranged from 10 to 45 years (32.00 ± 15.64 years). The disease duration until the last examination ranged from 7 to 16 years (10.00 ± 3.46 years). Most patients presented cerebellar signs such as gait disturbances, clumsy limbs, dysarthria, and dysfunction of ocular movement. Dysfunction of ocular movement presented as limitation of ocular movement and dysmetria of the saccade. Muscle strength remained normal during the disease course in all patients. Cerebellar atrophy on brain MRI was found in 5/6 patients (four with marked atrophy, one with moderate atrophy, and one with pontine ‘hot cross bun’ sign) (Fig. 1d). Cognitive impairment, anxiety, and depression were identified in some patients. Neurophysiologic examinations were performed in four patients, and no abnormalities were identified.
Cases with MSA and PKD symptoms
Patient 1 (F2: II-1) is a 58-year-old woman who presented with progressive gait instability, incoordination of limbs, and dysphagia at the age of 50. The patient also complained of frequent urination and constipation. She had no evident family history. Neurological examination revealed lingual tremor, severe hypokinetic dysarthria, diffuse hypertonia, and incoordination of limb movements. Lightheadedness was observed when standing up, and orthostatic hypotension was detected. Brain MRI demonstrated marked cerebellar and pontine atrophy and pontine ‘hot cross bun’ sign (Fig. 1d). The patient was clinically diagnosed with probable MSA-C and was also shown to carry CTA/CTG expansion.
Patient 2 (F6: III-1) is a 26-year-old man presenting with progressive ataxia accompanied by PKD phenotype with onset by the age of ten. He presented with recurrent and brief attacks of rigidity and numbness of left limbs triggered by sudden voluntary movement, lasting for about 10 s and with a frequency of 3–4 times per week. He also developed progressive gait ataxia and chorea. He moved with a wide-based gait and had difficulty with tandem gait. Clinical examination revealed hyperreflexia and hypertonia of lower limbs. Unfortunately, we were unable to capture an attack of rigidity triggered during the outpatient examination. Although the attack was not witnessed in hospital, the symptoms were validated by the patient several times in the follow-up visit. MRI of the brain and thoracic spinal cord and electroencephalogram were all normal. According to WES, we did not observe PRRT2 or other paroxysmal disease-related pathogenic mutations. The rare variants identified in the patient are described in Supplementary Table 3. The patient was prescribed carbamazepine (0.1 g, tid), but it was refused in the follow-up visit due to side effects upon introduction.
Discussion
SCA8 is a unique SCA subtype characterized by slowly progressive ataxia, associated with additional features such as pyramidal and extrapyramidal symptoms, cognitive impairment, and psychiatric abnormalities. In our research, we identified six ataxia patients with pathogenic CTA/CTG repeat expansion in the ATXN8/ATXN8OS gene, with no evidence of expanded alleles in healthy controls. Patients with CTA/CTG expansion accounted for approximately 1.66% (6/362) of patients without definite molecular diagnosis. This is the largest scale study to analyze the association between CTA/CTG expansion and ataxia syndromes in mainland China. Our results indicated that in mainland China, SCA8 repeat expansion is worthy of investigation in ataxia patients after exclusion of the common SCA subtypes, especially in sporadic patients with slow progression. We did not analyze the interruptions within the CTG tract, as the estimate for pathogenic CTA/CTG repeats depends only on repeat length. As reported previously, almost all CTA/CTG repeat tracts with fewer than 50 repeats do not have interruptions, while pure and interrupted expansion can both be found in SCA8 patients with > 80 CTA/CTG repeats; interruption may play some role in penetrance [10]. Incomplete penetrance was also observed in this study. None of the six patients had a known family history. Family 1 exhibited paternal genetic transmission, with a decrease of 63 repeats. In family 5, maternal transmission was observed with a decrease of 97 repeats. However, although the mother and father of the probands carried CTA/CTG expansions, they did not show ataxia at the last neurological examination. In our cohort, the penetrance was lower than that previously reported, which might be explained by different backgrounds or modifying factors. The low penetrance makes it difficult for genetic counseling clinically, therefore, should be informed to the relatives and offspring of the patients with repeat expansions.
The frequency and phenotype of reported SCA8 patients vary in different regions [9]. The highest frequency of 5.9% was observed in Finland, while the frequency was 1.66% in our cohort. According to the literature, approximately 80–90% of SCA8 cases present with dysarthria, and about 30–50% of SCA8 patients present with nystagmus and sensory signs [9]. However, in our study, nystagmus and sensory signs were not observed, and dysarthria was only found in 4/6 patients. Cognitive impairment was found in 30% of patients (2/6), consistent with the literature [9]. Three affected patients showed either clinically significant or borderline levels of anxiety and depression, which were also reported in America and European patients [7, 14].
SCA8 has been reported to have high clinical heterogeneity. Besides ataxia, some patients also exhibit cognitive, psychiatric dysfunction, multisystem atrophy (MSA), and other neurodegenerative disease phenotypes. Moreover, CTA/CTG expansion has also been detected in MSA [27], Parkinson’s disease [23], Alzheimer’s disease [28], amyotrophic lateral sclerosis [29], and psychosis [30]. In this study, two specific phenotypes, PKD and MSA, were found to be associated with CTA/CTG expansion. One patient with CTA/CTG expansion and symptoms suggestive of PKD phenotype was described. The PRRT2 and other paroxysmal disease pathogenic mutations were excluded by WES. To the best of our knowledge, this is the first report describing the PKD phenotype associated with CTA/CTG expansion, extending the clinical spectrum of SCA8. It should be noted that our finding is not coincidental, as an episodic course has also previously been described in a few cases. For example, Gupta et al. reported SCA8 cases with episodic ataxia, migraines, and other fluctuating symptoms [9]. Some experimental research has also elucidated the episodic course. The expanded RNA transcript encoded by ATXN8OS is suspected to regulate the expression of the KLHL1 gene [31, 32], which may regulate ion channel location and signaling, and is reportedly associated with cerebellar symptoms in animal models [33, 34]. Ion channel deficiency is involved in the pathogenesis of SCA symptoms and episodic courses, such as SCA6 and episodic ataxia type 2 [35]. In light of these observations, we suggest that the CTA/CTG expansion in the ATXN8/ATXN8OS gene might play an important role in the molecular mechanism of episodic symptoms. PKD phenotypes might be atypical clinical features of SCA8, but more research is required to investigate the underlying mechanism.
Besides PKD, we also described a patient carrying the CTA/CTG expansion with an MSA-C phenotype. In fact, some patients with a clinical diagnosis of probable MSA-C and a proven CTA/CTG expansion have already been reported [8, 27, 36]. However, some studies have failed to identify the CTA/CTG expansion in MSA-C patients [28, 37], and the CTA/CTG expansion has also been detected in healthy controls. Therefore, considering the high genetic heterogeneity of SCA8, our research provides more evidence that multiple system atrophy might be an atypical symptom of SCA8.
In summary, SCA8 is a cerebellar disease with a high degree of clinical and genetic heterogeneity. We described the genetic and clinical features of SCA8 patients from mainland China and confirmed the association between the CTA/CTG expansion and MSA-C phenotype. For the first time, we suggest that CTA/CTG expansion may be associated with a PKD phenotype, and further investigations are needed to better understand the role of CTA/CTG repeat expansion in the underlying mechanism.
References
Durr A (2010) Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol 9:885–894. https://doi.org/10.1016/s1474-4422(10)70183-6
Koob MD, Moseley ML, Schut LJ, Benzow KA, Bird TD, Day JW, Ranum LP (1999) An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet 21:379–384. https://doi.org/10.1038/7710
Day JW, Schut LJ, Moseley ML, Durand AC, Ranum LP (2000) Spinocerebellar ataxia type 8: clinical features in a large family. Neurology 55:649–657
Zeman A (2004) Spinocerebellar ataxia type 8 in Scotland: genetic and clinical features in seven unrelated cases and a review of published reports. J Neurol Neurosurg Psychiatr 75:459–465. https://doi.org/10.1136/jnnp.2003.018895
Felling RJ, Barron TF (2005) Early onset of ataxia in a child with a pathogenic SCA8 allele. Pediatr Neurol 33:136–138. https://doi.org/10.1016/j.pediatrneurol.2005.02.010
Lilja A, Hamalainen P, Kaitaranta E, Rinne R (2005) Cognitive impairment in spinocerebellar ataxia type 8. J Neurol Sci 237:31–38. https://doi.org/10.1016/j.jns.2005.05.008
Torrens L, Burns E, Stone J, Graham C, Wright H, Summers D, Sellar R, Porteous M, Warner J, Zeman A (2008) Spinocerebellar ataxia type 8 in Scotland: frequency, neurological, neuropsychological and neuropsychiatric findings. Acta Neurol Scand 117:41–48. https://doi.org/10.1111/j.1600-0404.2007.00904.x
Munhoz RP, Teive HA, Raskin S, Werneck LC (2009) CTA/CTG expansions at the SCA 8 locus in multiple system atrophy. Clin Neurol Neurosurg 111:208–210. https://doi.org/10.1016/j.clineuro.2008.09.003
Gupta A, Jankovic J (2009) Spinocerebellar ataxia 8: variable phenotype and unique pathogenesis. Parkinsonism Relat Disord 15:621–626. https://doi.org/10.1016/j.parkreldis.2009.06.001
Moseley ML, Schut LJ, Bird TD, Koob MD, Day JW, Ranum LP (2000) SCA8 CTG repeat: en masse contractions in sperm and intergenerational sequence changes may play a role in reduced penetrance. Hum Mol Genet 9:2125–2130
Vincent JB, Neves-Pereira ML, Paterson AD, Yamamoto E, Parikh SV, Macciardi F, Gurling HM, Potkin SG, Pato CN, Macedo A, Kovacs M, Davies M, Lieberman JA, Meltzer HY, Petronis A, Kennedy JL (2000) An unstable trinucleotide-repeat region on chromosome 13 implicated in spinocerebellar ataxia: a common expansion locus. Am J Hum Genet 66:819–829. https://doi.org/10.1086/302803
Moseley ML, Zu T, Ikeda Y, Gao W, Mosemiller AK, Daughters RS, Chen G, Weatherspoon MR, Clark HB, Ebner TJ, Day JW, Ranum LP (2006) Bidirectional expression of CUG and CAG expansion transcripts and intranuclear polyglutamine inclusions in spinocerebellar ataxia type 8. Nat Genet 38:758–769. https://doi.org/10.1038/ng1827
Juvonen V, Hietala M, Paivarinta M, Rantamaki M, Hakamies L, Kaakkola S, Vierimaa O, Penttinen M, Savontaus ML (2000) Clinical and genetic findings in Finnish ataxia patients with the spinocerebellar ataxia 8 repeat expansion. Ann Neurol 48:354–361
Ikeda Y, Dalton JC, Moseley ML, Gardner KL, Bird TD, Ashizawa T, Seltzer WK, Pandolfo M, Milunsky A, Potter NT, Shoji M, Vincent JB, Day JW, Ranum LP (2004) Spinocerebellar ataxia type 8: molecular genetic comparisons and haplotype analysis of 37 families with ataxia. Am J Hum Genet 75:3–16. https://doi.org/10.1086/422014
Silveira I, Alonso I, Guimaraes L, Mendonca P, Santos C, Maciel P, Fidalgo De Matos JM, Costa M, Barbot C, Tuna A, Barros J, Jardim L, Coutinho P, Sequeiros J (2000) High germinal instability of the (CTG)n at the SCA8 locus of both expanded and normal alleles. Am J Hum Genet 66:830–840. https://doi.org/10.1086/302827
Cellini E, Nacmias B, Forleo P, Piacentini S, Guarnieri BM, Serio A, Calabro A, Renzi D, Sorbi S (2001) Genetic and clinical analysis of spinocerebellar ataxia type 8 repeat expansion in Italy. Arch Neurol 58:1856–1859
Cintra VP, Lourenço CM, Marques SE, de Oliveira LM, Tumas V, Marques W (2014) Mutational screening of 320 Brazilian patients with autosomal dominant spinocerebellar ataxia. J Neurol Sci 347:375–379. https://doi.org/10.1016/j.jns.2014.10.036
Topisirovic I, Dragasevic N, Savic D, Ristic A, Keckarevic M, Keckarevic D, Culjkovic B, Petrovic I, Romac S, Kostic VS (2002) Genetic and clinical analysis of spinocerebellar ataxia type 8 repeat expansion in Yugoslavia. Clin Genet 62:321–324
Musova Z, Sedlacek Z, Mazanec R, Klempir J, Roth J, Plevova P, Vyhnalek M, Kopeckova M, Apltova L, Krepelova A, Zumrova A (2013) Spinocerebellar ataxias type 8, 12, and 17 and dentatorubro-pallidoluysian atrophy in Czech ataxic patients. Cerebellum 12:155–161. https://doi.org/10.1007/s12311-012-0403-5
Hu Y, Hashimoto Y, Ishii T, Rayle M, Soga K, Sato N, Okita M, Higashi M, Ozaki K, Mizusawa H, Ishikawa K, Yokota T (2017) Sequence configuration of spinocerebellar ataxia type 8 repeat expansions in a Japanese cohort of 797 ataxia subjects. J Neurol Sci 382:87–90. https://doi.org/10.1016/j.jns.2017.08.3256
Wang M, Guo S, Yao W, Wang J, Tao J, Zhou Y, Ying B (2018) Identification of abnormal 51 CTA/CTG expansion as probably the shortest pathogenic allele for spinocerebellar ataxia-8 in China. Neurosci Bull 34:859–862. https://doi.org/10.1007/s12264-018-0247-1
Soong BW, Lu YC, Choo KB, Lee HY (2001) Frequency analysis of autosomal dominant cerebellar ataxias in Taiwanese patients and clinical and molecular characterization of spinocerebellar ataxia type 6. Arch Neurol 58:1105–1109
Wu YR, Chen IC, Soong BW, Kao SH, Lee GC, Huang SY, Fung HC, Lee-Chen GJ, Chen CM (2009) SCA8 repeat expansion: large CTA/CTG repeat alleles in neurological disorders and functional implications. Hum Genet 125:437–444. https://doi.org/10.1007/s00439-009-0641-x
Harding AE (1993) Clinical features and classification of inherited ataxias. Adv Neurol 61:1–14
Krysa W, Rajkiewicz M, Sutek A (2012) Rapid detection of large expansions in progressive myoclonus epilepsy type 1, myotonic dystrophy type 2 and spinocerebellar ataxia type 8. Neurologia i Neurochirurgia Polska 46:113–120. https://doi.org/10.5114/ninp.2012.28253
Wang JL, Cao L, Li XH, Hu ZM, Li JD, Zhang JG, Liang Y, San A, Li N, Chen SQ, Guo JF, Jiang H, Shen L, Zheng L, Mao X, Yan WQ, Zhou Y, Shi YT, Ai SX, Dai MZ, Zhang P, Xia K, Chen SD, Tang BS (2011) Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain 134:3493–3501. https://doi.org/10.1093/brain/awr289
Smetcoren C, Weckhuysen D (2016) SCA 8 mimicking MSA-C. Acta Neurol Belg 116:221–222. https://doi.org/10.1007/s13760-015-0523-z
Sobrido MJ, Cholfin JA, Perlman S, Pulst SM, Geschwind DH (2001) SCA8 repeat expansions in ataxia: a controversial association. Neurology 57:1310–1312
Hirano M, Samukawa M, Isono C, Saigoh K, Nakamura Y, Kusunoki S (2018) Noncoding repeat expansions for ALS in Japan are associated with the ATXN8OS gene. Neurol Genet 4:e252. https://doi.org/10.1212/nxg.0000000000000252
Vincent JB, Yuan QP, Schalling M, Adolfsson R, Azevedo MH, Macedo A, Bauer A, DallaTorre C, Medeiros HM, Pato MT, Pato CN, Bowen T, Guy CA, Owen MJ, O’Donovan MC, Paterson AD, Petronis A, Kennedy JL (2000) Long repeat tracts at SCA8 in major psychosis. Am J Med Genet 96:873–876
Ikeda Y, Daughters RS, Ranum LP (2008) Bidirectional expression of the SCA8 expansion mutation: one mutation, two genes. Cerebellum 7:150–158. https://doi.org/10.1007/s12311-008-0010-7
Chen WL, Lin JW, Huang HJ, Wang SM, Su MT, Lee-Chen GJ, Chen CM, Hsieh-Li HM (2008) SCA8 mRNA expression suggests an antisense regulation of KLHL1 and correlates to SCA8 pathology. Brain Res 1233:176–184. https://doi.org/10.1016/j.brainres.2008.07.096
He Y, Zu T, Benzow KA, Orr HT, Clark HB, Koob MD (2006) Targeted deletion of a single Sca8 ataxia locus allele in mice causes abnormal gait, progressive loss of motor coordination, and Purkinje cell dendritic deficits. J Neurosci 26:9975–9982. https://doi.org/10.1523/jneurosci.2595-06.2006
Aromolaran KA, Benzow KA, Koob MD, Piedras-Renteria ES (2007) The Kelch-like protein 1 modulates P/Q-type calcium current density. Neuroscience 145:841–850. https://doi.org/10.1016/j.neuroscience.2006.12.046
Bushart DD, Shakkottai VG (2019) Ion channel dysfunction in cerebellar ataxia. Neurosci Lett 688:41–48. https://doi.org/10.1016/j.neulet.2018.02.005
Factor SA, Qian J, Lava NS, Hubbard JD, Payami H (2005) False-positive SCA8 gene test in a patient with pathologically proven multiple system atrophy. Ann Neurol 57:462–463. https://doi.org/10.1002/ana.20389
Schols L, Szymanski S, Peters S, Przuntek H, Epplen JT, Hardt C, Riess O (2000) Genetic background of apparently idiopathic sporadic cerebellar ataxia. Hum Genet 107:132–137
Li J, Zhao T, Zhang Y, Zhang K, Shi L, Chen Y, Wang X, Sun Z (2018) Performance evaluation of pathogenicity-computation methods for missense variants. Nucleic Acids Res 46:7793–7804. https://doi.org/10.1093/nar/gky678
Acknowledgements
We are grateful to the participating patients for their involvement. This work was supported by the National Key Research and Development Program of China (#2018YFC1312003, 2016YFC1306000); the Program of National Natural Science Foundation of China (#81671120, 81300981, 81430023, 81771231); the Clinical Scientific program of Xiangya Hospital, Central South University (#2015105); and Innovation Driven Program of Central South University (#506010108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
This study was approved by the Ethics Committee and the Expert Committee of Xiangya Hospital, Central South University (equivalent to an Institutional Review Board).
Informed consent
Informed consent was obtained in writing from all participants.
Electronic supplementary material
Below is the link to the electronic supplementary material. Supplementary Table 3. List of variants identified by whole exome sequencing in the patient with PKD phenotype. The criteria for filtering: (1) dominant pattern: heterozygous variants (MAF ≤ 0.001). (2) recessive pattern: homozygous variants and compound heterozygous variants (MAF ≤ 0.01). In silico analysis: (1) stop gain/loss, frameshift and splice site mutations: falling within two base pairs of exon–intron junctions; (2) missense variants functionally predicted to be deleterious (REVE > 0.7). MAF minor allele frequency, REVE [38] combination of REVEL and VEST3.
Rights and permissions
About this article
Cite this article
Zhou, Y., Yuan, Y., Liu, Z. et al. Genetic and clinical analyses of spinocerebellar ataxia type 8 in mainland China. J Neurol 266, 2979–2986 (2019). https://doi.org/10.1007/s00415-019-09519-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09519-2