Abstract
Background
Atypical cerebrospinal fluid (CSF) patterns, involving an increase in the concentration of phosphorylated-tau (P-tau) proteins but normal amyloid-β concentration, are not uncommon in patients with mild neurocognitive disorders and suspected Alzheimer’s disease (AD). In these conditions, however, AD diagnosis may be ruled out in the absence of any amyloid deposition at positron-emission tomography (PET). This pilot cross-sectional study was aimed to determine whether this negativity of amyloid PET can be predicted by CSF profiles in such patients.
Methods
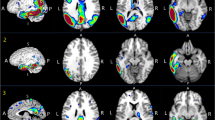
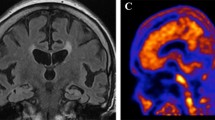
Twenty-five patients (73 [68–80] years, 10 women) with mild neurocognitive disorders, suspected AD and an increase in the CSF concentration of P-tau proteins but normal Aβ42 concentration and Aβ42/Aβ40 ratio were prospectively included and referred to a 18F-florbetaben PET. The latter was considered as definitively negative with the conjunction of both visual (brain amyloid plaque load score) and quantified (standard uptake value ratios) criteria. Predictors of a negative PET were searched among current CSF biomarkers (Aß42, Aß40, T-tau, P-tau, Aß42/Aß40, Aß42/p-tau).
Results
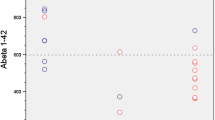
Amyloid PET was negative in 15 patients (60%) with a CSF Aß42 concentration being the sole independent predictor of this negativity. The criterion of an Aß42 concentration in the very high range (> 843 pg/mL), observed in 60% (15/25) of the study patients, was associated with a negative amyloid PET in 93% (14/15) of cases.
Conclusions
In mild neurocognitive disorders patients with suspected AD and showing an increase in CSF P-tau protein level, amyloid PETs are commonly negative, when Aß42 concentration is in the very high range. In such case, AD diagnosis based on biomarkers can be ruled out with reasonable certainty, without the need for additional CSF second-line assays or results from amyloid PET.


Similar content being viewed by others
References
Jack CR, Bennett DA, Blennow K et al (2018) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 14:535–562. https://doi.org/10.1016/j.jalz.2018.02.018
Koopman K, Le Bastard N, Martin J-J et al (2009) Improved discrimination of autopsy-confirmed Alzheimer’s disease (AD) from non-AD dementias using CSF P-tau (181P). Neurochem Int 55:214–218. https://doi.org/10.1016/j.neuint.2009.02.017
Sauvée M, DidierLaurent G, Latarche C et al (2014) Additional use of Aβ42/Aβ40 ratio with cerebrospinal fluid biomarkers P-tau and Aβ42 increases the level of evidence of Alzheimer’s disease pathophysiological process in routine practice. J Alzheimers Dis JAD 41:377–386. https://doi.org/10.3233/JAD-131838
Gabelle A, Dumurgier J, Vercruysse O et al (2013) Impact of the 2008–2012 French Alzheimer plan on the use of cerebrospinal fluid biomarkers in research memory center: the PLM Study. J Alzheimers Dis JAD 34:297–305. https://doi.org/10.3233/JAD-121549
Sabri O, Sabbagh MN, Seibyl J et al (2015) Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer’s disease: phase 3 study. Alzheimers Dement J Alzheimers Assoc 11:964–974. https://doi.org/10.1016/j.jalz.2015.02.004
Sabri O, Seibyl J, Rowe C, Barthel H (2015) Beta-amyloid imaging with florbetaben. Clin Transl Imaging 3:13–26. https://doi.org/10.1007/s40336-015-0102-6
Pontecorvo MJ, Arora AK, Devine M et al (2017) Quantitation of PET signal as an adjunct to visual interpretation of florbetapir imaging. Eur J Nucl Med Mol Imaging 44:825–837. https://doi.org/10.1007/s00259-016-3601-4
Harn NR, Hunt SL, Hill J et al (2017) Augmenting amyloid PET interpretations with quantitative information improves consistency of early amyloid detection. Clin Nucl Med 42:577–581. https://doi.org/10.1097/RLU.0000000000001693
Thurfjell L, Lilja J, Lundqvist R et al (2014) Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med 55:1623–1628. https://doi.org/10.2967/jnumed.114.142109
Nayate AP, Dubroff JG, Schmitt JE et al (2015) Use of standardized uptake value ratios decreases interreader variability of [18F] florbetapir PET brain scan interpretation. AJNR Am J Neuroradiol 36:1237–1244. https://doi.org/10.3174/ajnr.A4281
Bullich S, Seibyl J, Catafau AM et al (2017) Optimized classification of18F-Florbetaben PET scans as positive and negative using an SUVR quantitative approach and comparison to visual assessment. NeuroImage Clin 15:325–332. https://doi.org/10.1016/j.nicl.2017.04.025
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub, Washington DC
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology 34:939–944
Gervaise-Henry C, Watfa G, Albuisson E et al (2017) Cerebrospinal fluid Aβ42/Aβ40 as a means to limiting tube- and storage-dependent pre-analytical variability in clinical setting. J Alzheimers Dis JAD 57:437–445. https://doi.org/10.3233/JAD-160865
Manca C, Hopes L, Kearney-Schwartz A et al (2019) Assessment of 18F-florbetaben amyloid PET imaging in patients with suspected Alzheimer’s disease and isolated increase in cerebrospinal fluid tau proteins. J Alzheimers Dis. https://doi.org/10.3233/JAD-181146
Dumurgier J, Vercruysse O, Paquet C et al (2013) Intersite variability of CSF Alzheimer’s disease biomarkers in clinical setting. Alzheimers Dement 9:406–413. https://doi.org/10.1016/j.jalz.2012.06.006
Lehmann S, Schraen S, Quadrio I et al (2014) Impact of harmonization of collection tubes on Alzheimer’s disease diagnosis. Alzheimers Dement J Alzheimers Assoc 10:S390–S394. https://doi.org/10.1016/j.jalz.2013.06.008
Chetouani A, Chawki MB, Hossu G et al (2018) Cross-sectional variations of white and grey matter in older hypertensive patients with subjective memory complaints. NeuroImage Clin 17:804–810. https://doi.org/10.1016/j.nicl.2017.12.024
Presotto L, Iaccarino L, Sala A et al (2018) Low-dose CT for the spatial normalization of PET images: a validation procedure for amyloid-PET semi-quantification. NeuroImage Clin 20:153–160. https://doi.org/10.1016/j.nicl.2018.07.013
Catafau AM, Bullich S, Seibyl JP et al (2016) Cerebellar amyloid-β plaques: how frequent are they, and do they influence 18F-florbetaben SUV ratios? J Nucl Med 57:1740–1745. https://doi.org/10.2967/jnumed.115.171652
Barthel H, Gertz H-J, Dresel S et al (2011) Cerebral amyloid-β PET with florbetaben (18F) in patients with Alzheimer’s disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol 10:424–435. https://doi.org/10.1016/S1474-4422(11)70077-1
Morris JC (1993) The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43:2412–2414
Fagan AM, Mintun MA, Mach RH et al (2006) Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol 59:512–519. https://doi.org/10.1002/ana.20730
Forsberg A, Engler H, Almkvist O et al (2008) PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging 29:1456–1465. https://doi.org/10.1016/j.neurobiolaging.2007.03.029
Grimmer T, Riemenschneider M, Förstl H et al (2009) Beta amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry 65:927–934. https://doi.org/10.1016/j.biopsych.2009.01.027
Tolboom N, van der Flier WM, Yaqub M et al (2009) Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med 50:1464–1470. https://doi.org/10.2967/jnumed.109.064360
Jagust WJ, Landau SM, Shaw LM et al (2009) Relationships between biomarkers in aging and dementia. Neurology 73:1193–1199. https://doi.org/10.1212/WNL.0b013e3181bc010c
Landau SM, Lu M, Joshi AD et al (2013) Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann Neurol 74:826–836. https://doi.org/10.1002/ana.23908
Tapiola T, Alafuzoff I, Herukka S-K et al (2009) Cerebrospinal fluid β-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66:382–389. https://doi.org/10.1001/archneurol.2008.596
Lebouvier T, Pasquier F, Buée L (2017) Update on tauopathies. Curr Opin Neurol 30:589–598. https://doi.org/10.1097/WCO.0000000000000502
Vlassenko AG, McCue L, Jasielec MS et al (2016) Imaging and cerebrospinal fluid biomarkers in early preclinical alzheimer disease. Ann Neurol 80:379–387. https://doi.org/10.1002/ana.24719
Acknowledgements
The authors thank Pierre Pothier for critical review of the manuscript.
Funding
This work was carried out thanks to the support of the Nancyclotep Imaging Platform and Nancy University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
The study was approved by the French ethics committee CPP Est III on September 15th, 2015, as well as received the authorization from the national competent authority (ANSM) on September 18th 2015, and adhered to the Declaration of Helsinki.
Informed consent
All patients provided written informed consent for participation in the study. All patients provided written informed consent for publication.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manca, C., Rivasseau Jonveaux, T., Roch, V. et al. Amyloid PETs are commonly negative in suspected Alzheimer’s disease with an increase in CSF phosphorylated-tau protein concentration but an Aβ42 concentration in the very high range: a prospective study. J Neurol 266, 1685–1692 (2019). https://doi.org/10.1007/s00415-019-09315-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09315-y