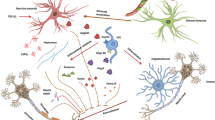
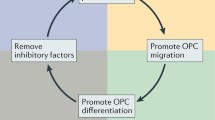
Abstract
Historically considered to be an autoimmune demyelinating disease, multiple sclerosis is now recognized to be characterized by significant axonal and neuronal pathology. Addressing this neurodegenerative component of the disease is an important treatment objective, since axonal injury is believed to underlie the accumulation of disability and disease progression. The precise relationship between the inflammatory and neurodegenerative components in multiple sclerosis remains poorly elucidated, although neurodegeneration appears to be at least partially independent from neuroinflammation. The mechanisms underlying axonal injury appear complex and are likely to be multifactorial. Specific treatment strategies need to be developed that act within the central nervous system to prevent neurodegeneration and need to be provided from the earliest stages of disease. It is likely that immunomodulatory treatments acting purely in the periphery will provide only indirect and not direct neuroprotection. A promising approach is to enhance neuroprotective autoimmunity inside the brain, believed to be mediated, at least in part, by the release of neurotrophic factors within the nervous system from infiltrating immune cells. Such a beneficial process would be inhibited by a non-selective immunosuppressive strategy. In summary, treatments of multiple sclerosis should take into account the heterogeneous pathophysiology of the disease. The pathogenic process in the central nervous system itself should be the major focus in multiple sclerosis therapy in order to protect against demyelination and axonal loss and to promote remyelination and regeneration directly in the target tissue, independently of peripheral immune status. In conclusion, selective treatment strategies aimed at preventing axonal injury within the central nervous system are required to complement existing, peripherally acting treatments targeting the immune system.
Similar content being viewed by others
References
Arredondo LR, Deng C, Ratts RB, et al. (2001) Role of nerve growth factor in experimental autoimmune encephalomyelitis. Eur J Immunol 31:625–633
Besser M, Wank R (1999) Cutting edge: clonally restricted production of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3 mRNA by human immune cells and Th1/Th2-polarized expression of their receptors. J Immunol 162:6303–6306
Bitsch A, Schuchardt J, Bunkowski S, et al. (2000) Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain 123:1174–1183
Bo L, Vedeler CA, Nyland H, et al. (2003) Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult Scler 9:323–331
Braun A, Lommatzsch M, Mannsfeldt A, et al. (1999) Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am J Respir Cell Mol Biol 21:537–546
Brundula V, Rewcastle NB, Metz LM, et al. (2002) Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain 125:1297–1308
Butzkueven H, Zhang JG, Soilu-Hanninen M, et al. (2002) LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med 8:613–619
Cannella B, Hoban CJ, Gao YL, et al. (1998) The neuregulin, glial growth factor 2, diminishes autoimmune demyelination and enhances remyelination in a chronic relapsing model for multiple sclerosis. Proc Nat Acad Sci USA 95:10100–10105
Cannella B, Pitt D, Capello E, Raine CS (2000) Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination. Am J Pathol 157:933–943
Chen M, Valenzuela RM, Dhib-Jalbut S (2003) Glatiramer acetate-reactive T cells produce brain-derived neurotrophic factor. J Neurol Sci 215:37–44
Chitnis T, Imitola J, Khoury SJ (2005) Therapeutic strategies to prevent neurodegeneration and promote regeneration in multiple sclerosis. Curr Drug Targets Immune Endocr Metabol Disord 5:11–26
Coles AJ, Wing MG, Molyneux P, et al. (1999) Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 46:296–304
Compston A, Coles A (2002) Multiple sclerosis. Lancet 359:1221–1231
Filippi M, Bozzali M, Rovaris M, et al. (2003) Evidence for widespread axonal damage at the earliest clinical stage of multiple sclerosis. Brain 126:433–437
Flügel A, Matsumuro K, Neumann H, et al. (2001) Anti-inflammatory activity of nerve growth factor in experimental autoimmune encephalomyelitis: inhibition of monocyte transendothelial migration. Eur J Immunol 31:11–22
Giuliani F, Yong VW (2003) Immunemediated neurodegeneration and neuroprotection in MS. Int MS J 10:122–130
Gravel C, Gotz R, Lorrain A, Sendtner M (1997) Adenoviral gene transfer of ciliary neurotrophic factor and brain-derived neurotrophic factor leads to long-term survival of axotomized motor neurons. Nat Med 3:765–770
Grigoriadis N, Ben-Hur T, Karussis D, Milonas I (2004) Axonal damage in multiple sclerosis: a complex issue in a complex disease. Clin Neurol Neurosurg 106:211–217
Kerschensteiner M, Gallmeier E, Behrens L, et al. (1999) Activated human T cells, B cells and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med 189:865–870
Kerschensteiner M, Stadelmann C, Dechant G, et al. (2003) Neurotrophic cross-talk between the nervous and immune systems: implications for neurological diseases. Ann Neurol 53:292–304
Kieseier BC, Hartung HP (2003) Multiple paradigm shifts in multiple sclerosis. Curr Opin Neurol 16:247–252
Korner H, Goodsall AL, Lemckert FA, et al. (1995) Unimpaired autoreactive T cell traffic within the central nervous system during tumor necrosis factor receptor-mediated inhibition of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci 92:11066–11070
Korner H, Lemckert FA, Chaudhri G, et al. (1997) Tumor necrosis factor blockade in actively induced experimental autoimmune encephalomyelitis prevents clinical disease despite activated T cell infiltration to the central nervous system. Eur J Immunol 27:1973–1981
Liu X, Linnington C, Webster HD, et al. (1997) Insulin-like growth factor-I treatment reduces immune cell responses in acute non-demyelinative experimental autoimmune encephalomyelitis. J Neurosci Res 47:531–538
Lovett-Racke AE, Bittner P, Cross AH, et al. (1998) Regulation of experimental autoimmune encephalomyelitis with insulin-like growth factor (IGF-1) and IGF-1/IGF-binding protein-3 complex (IGF-1/IGFBP3). J Clin Invest 101:1797–1804
Metz LM, Zhang Y, Yeung M, et al. (2004) Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 55:756
Moalem G, Leibowitz-Amit R, Yoles E, et al. (1999) Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med 5:49–55
Neuhaus O, Farina C, Wekerle H, Hohlfeld R (2001) Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology 56:702–708
Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG (2000) Multiple sclerosis. N Engl J Med 343:938–952
Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50:389–400
Popovic N, Schubart A, Goetz BD, et al. (2002) Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol 51:215–223
Schwartz M, Kipnis J (2005) Protective autoimmunity and neuroprotection in inflammatory and noninflammatory neurodegenerative diseases. J Neurol Sci 233:163–166
Stadelmann C, Kerschensteiner M, Misgeld T, et al. (2002) BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain 125:75–85
Steinman L (2001) Multiple sclerosis: a two-stage disease. Nat Immunol 2:762–764
Teismann P, Schulz JB (2004) Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell Tissue Res 318:149–161
The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group (1999) TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53:457–465
Tikka T, Fiebich BL, Goldsteins G, et al. (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 21:2580–2588
Villoslada P, Hauser SL, Bartke I, et al. (2000) Human nerve growth factor protects common marmosets against autoimmune encephalomyelitis by switching the balance of T helper cell type 1 and 2 cytokines within the central nervous system. J Exp Med 191:1799–1806
Wiendl H, Hohlfeld R (2002) Therapeutic approaches in multiple sclerosis: lessons from failed and interrupted treatment trials. BioDrugs 16:183–200
Ziemssen T, Kumpfel T, Klinkert WE, et al. (2002) Glatiramer acetate-specific T-helper 1- and 2-type cell lines produce BDNF: implications for multiple sclerosis therapy. Brain 125:2381–2391
Ziemssen T (2004) Neuroprotection and glatiramer acetate: the possible role in the treatment of multiple sclerosis. Adv Exp Med Biol 541:111–134
Ziemssen T, Ziemssen F (2005) The role of the humanal immune system in multiple sclerosis (MS) and its animal model experimental automimmune encephalomyelitis (EAE). Autoimmun Rev 4:460–467
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ziemssen, T. Modulating processes within the central nervous system is central to therapeutic control of multiple sclerosis. J Neurol 252 (Suppl 5), v38–v45 (2005). https://doi.org/10.1007/s00415-005-5007-2
Issue Date:
DOI: https://doi.org/10.1007/s00415-005-5007-2