Abstract
“Severe acute respiratory syndrome” (SARS) due to coronavirus (SARS-CoV-2) infection is a well-known cause of death. Sometimes, demise can occur unexpectedly in apparently previous healthy individual after a brief period of trivial flu-like symptoms. In these doubtful cases, the forensic pathologist could be requested to define the cause of death occurred outside the hospital. In this report, the authors describe two autopsied cases of SARS-CoV-2-related deaths which occurred suddenly at home and were not preceded by hospitalization, highlighting associated histopathologic patterns and correlating them to pathophysiology of viral infection.
Similar content being viewed by others
Introduction
The recent pandemic outbreak of SARS-CoV-2 (COVID-19) infection constitutes the leading health risk worldwide.
In Italy, during the first 4 months of 2020, especially among elderly and/or immunocompromised patients, SARS-CoV-2 has been responsible of a peak of morbidity and mortality, still developing today [1]. Furthermore, COVID-19 infection constitutes not only a cause of work overload but also a peculiar health risk for all healthcare staff.
On April 2020, the Italian Health Ministry had advised to avoid autopsy in case of comproved COVID-19-related death [2], depriving pathologists of a powerful tool for highlighting pathophysiology of SARS-CoV-2 infection [3].
Nevertheless, the forensic pathologist is not infrequently requested to autoptically define the cause of death occurred at home in doubtful cases, not immediately suspected to be SARS-CoV-2 related [4].
In the present report, the authors briefly describe two autoptic cases of SARS-CoV-2-related deaths. Autopsies were requested by the family physician in order to define the cause of unexpected demise of the two women, preceded only by a brief period of flu-like symptoms, which occurred suddenly at home and were not preceded by hospitalization or execution of nasopharyngeal swabs.
The aim of this report is to highlight histopathologic pattern detected at light microscopy (H&E) survey attempting to correlate them with conceivable pathophysiology of the COVID-19 viral attack.
Cases report
Forensic autopsies were performed on two Caucasian women, respectively, 67 and 61 years old, who suddenly died after 1 week of persistent flu-like symptoms accompanied by low-grade fever that does not require hospitalization.
Deaths occurred at home were preceded by sudden onset of a rapidly worsening dyspnea and anticipated intervention of the emergency staff.
The 61-year-old woman was affected by non-insulin-dependent diabetes mellitus and Hashimoto’s thyroiditis treated with daily low-dose corticosteroids.
Apparently no relevant disease affected the other patient.
Before beginning the autopsy, nasopharyngeal swabs were sampled and transferred to local pathoclinical laboratory. RT-PCR search for SARS-CoV-2-RNA gave positive results.
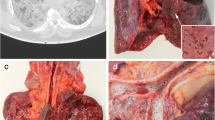
At autopsy, in both cases, the lungs appeared not particularly congested but heavy and hepatized, without macroscopic evidence of thromboembolism.
The 67-year-old woman showed mild splenomegaly (spleen weight 290 g).
No other macroscopic anomaly was found, in particular cardiac or encephalic.
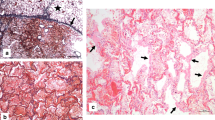
At light microscopy (H&E) survey, the lung parenchyma showed classic histopathologic pattern of diffuse alveolar damage (DAD): hyaline membranes plastered along the alveolar walls, type 2 pneumocytes hyperplasia, and arterial thrombi (Fig. 1).
Hyperplastic type 2 pneumocytes appeared marked atypical showing high nuclear to cytoplasmic ratio, bizarre eosinophilic nuclear inclusions seldom band-like, cytoplasmic accumulation of viral particles, cellular aggregation, or syncytial fusion (Fig. 2).
Type 2 pneumocytes atypical hyperplasia. a Highly atypical type 2 pneumocytes (note bizarre eosinophilic nuclear inclusions, seldom band-like) (H&E, × 1000, oil immersion). b and c Type 2 pneumocytes syncytial fusion (H&E; b, × 400; c, × 1000; oil immersion). d Type 2 pneumocytes engulfed perhaps of viral particles (H&E; d, × 1000, oil immersion)
Hyperplastic type 2 pneumocytes also contained Cowdry type A nuclear inclusions (Fig. 3).
Mild to moderate interstitial mononuclear infiltrations, intra-alveolar fine fibrin meshwork, or serous/hemorrhagic edema was seldom focally evident.
Organizing myxoid fibroblastic plugs and bronchial/bronchiolar inflammation was absent.
Naked megakaryocytes were overrepresented in lung capillaries and also clearly visible in the myocardial, glomerular, and adrenal cortex capillaries (Fig. 4).
Microscopic hemorrhagic foci were found in the adrenal cortex.
Expansion of red pulp and a severe reduction of white pulp were evident in the spleens. No germinal centers were detected.
Discussion
SARS is a potentially lethal viral lung acute disease, requiring intensive care especially mechanical ventilation.
SARS was reported for the first time during coronavirus (SARS-CoV-1) pandemia which occurred in 2002/2003 [5]. In that occasion, the histopathologic pattern associated with the clinical syndrome was defined as DAD, featured by hyaline membranes, type 2 pneumocytes hyperplasia, and distal arterial thrombi [6].
SARS is returned to be a leading cause of death during second 2020 coronavirus (SARS-CoV-2) pandemia.
Sometimes, demise occurs unexpectedly at home, after a brief period of trivial flu-like symptoms, fever, and sudden dyspnea without hospitalization or execution of nasopharyngeal swabs. Those unknown fatal cases request the intervention of the forensic pathologist.
The aim of the authors is to share histopathologic findings detected in the two autopsy cases of unsuspected acute fatal SARS due to SARS-CoV-2 infection, not altered by effects of mechanical ventilation or intensive pharmacological therapy, at the same time attempting to infer some insights into pathophysiology of viral attack.
In agreement to latest scientific publications [7, 8], it is immediately evident from our two autoptic cases that DAD still represents the main histopathologic background of SARS.
Microscopic topography of the lung damage shows that SARS-CoV-2 retains a selective tropism for type 1 pneumocytes: ubiquitous hyaline membranes and florid type 2 pneumocytes hyperplasia in response to extensive type 1 pneumocytes death. Also, type 2 pneumocytes are assaulted by virus as pointed out by unusual highly atypical reactive hyperplasia represented by enlarged cell with high nuclear to cytoplasmic ratio, bizarre eosinophilic nuclear inclusions, tendency to form cellular aggregates or syncytial fusion, and viral cytopathic effect (Cowdry type A inclusions).
No relevant interstitial inflammation, bronchitis/bronchiolitis, nor pneumonia was detected in the lung tissue.
Collaterally, SARS-CoV-2 infection elicits a release from bone marrow of megakaryocytes that embolize to the lung capillaries.
Single naked megakaryocytes are usual found within the pulmonary capillaries of patients with febrile illnesses, but, to the best of our knowledge, they are never been noted in other organs [9].
Megakaryocyte release induced by SARS-CoV-2 infection is striking because it is very conspicuous: released megakaryocytes were so many that they were able to filter through lung capillaries and to embolize further in other vital organs like the heart, kidney, and adrenal glands.
Since naked megakaryocytes in the bone marrow are typically associated to an increase of peripheral demand of thrombocytes, this storm of naked megakaryocytes means that SARS-CoV-2 could be able also to alter profoundly human coagulation system, as suggested by the formation of fibrin thrombi in the pulmonary distal arteries [10, 11].
In conclusion, histopathologic findings induce us to depict SARS-CoV-2 like a virus with peculiar ability to damage pneumocytes without eliciting an intense inflammatory response and at the same time a potential agent able to alter human coagulation system in a thrombophilic direction.
References
Istituto nazionale di Statistica (2020) Impatto dell’epidemia COVID-19 sulla mortalita’ totale della popolazione residente – primo trimestre 2020
Ministero della Salute (2020) “Indicazioni emergenziali connesse ad epidemia COVID-19 riguardanti il settore funebre, cimiteriale e di cremazione
Ledford H (2020) Autopsy slowdown hinders quest to determine how coronavirus kills. Nature. https://doi.org/10.1038/d41586-020-01355-z
Santurro A, Scopetti M, D'Errico S, Fineschi V (2020) A technical report from the Italian SARS-CoV-2 outbreak. Postmortem sampling and autopsy investigation in cases of suspected or probable COVID-19. Forensic Sci Med Pathol. https://doi.org/10.1007/s12024-020-00258-9
CDC (2003) Severe acute respiratory syndrome—Singapore. MMWR 52:405–411
Chong PY, Chui P, Ling AE, Franks TJ, Tai DY, Leo YS, Kaw GJ, Wansaicheong G, Chan KP, Ean Oon LL, Teo ES, Tan KB, Nakajima N, Sata T, Travis WD (2004) Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med 128(2):195–204
Hanley B, Lucas SB, Youd E, Swift B, Osborn M (2020) Autopsy in suspected COVID-19 cases. J Clin Pathol 73(5):239–242
Menter YT, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N, Frank S, Turek D, Willi N, Pargger H, Bassetti S, Leuppi JD, Cathomas G, Tolnay M, Mertz KD, Tzankov A (2020) Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. https://doi.org/10.1111/his.14134
Sharma GK, Talbot IC (1986) Pulmonary megakaryocytes: “missing link” between cardiovascular and respiratory disease? J Clin Pathol 39:969–976
Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S (2020) Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. https://doi.org/10.7326/M20-2003
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein et al (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2015432
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No ethical approval for this work was requested. The authors declare that the report does not include research involving human participants and/or animals. No informed consent was considered necessary.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tombolini, A., Scendoni, R. SARS-CoV-2-related deaths in routine forensic autopsy practice: histopathological patterns. Int J Legal Med 134, 2205–2208 (2020). https://doi.org/10.1007/s00414-020-02354-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-020-02354-5