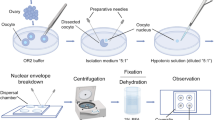
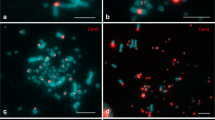
Abstract
In diplotene oocyte nuclei of all vertebrate species, except mammals, chromosomes lack interchromosomal contacts and chromatin is linearly compartmentalized into distinct chromomere-loop complexes forming lampbrush chromosomes. However, the mechanisms underlying the formation of chromomere-loop complexes remain unexplored. Here we aimed to compare somatic topologically associating domains (TADs), recently identified in chicken embryonic fibroblasts, with chromomere-loop complexes in lampbrush meiotic chromosomes. By measuring 3D-distances and colocalization between linear equidistantly located genomic loci, positioned within one TAD or separated by a TAD border, we confirmed the presence of predicted TADs in chicken embryonic fibroblast nuclei. Using three-colored FISH with BAC probes, we mapped equidistant genomic regions included in several sequential somatic TADs on isolated chicken lampbrush chromosomes. Eight genomic regions, each comprising two or three somatic TADs, were mapped to non-overlapping neighboring lampbrush chromatin domains — lateral loops, chromomeres, or chromomere-loop complexes. Genomic loci from the neighboring somatic TADs could localize in one lampbrush chromomere-loop complex, while genomic loci belonging to the same somatic TAD could be localized in neighboring lampbrush chromomere-loop domains. In addition, FISH-mapping of BAC probes to the nascent transcripts on the lateral loops indicates transcription of at least 17 protein-coding genes and 2 non-coding RNA genes during the lampbrush stage of chicken oogenesis, including genes involved in oocyte maturation and early embryo development.








Similar content being viewed by others
References
Ahmad MS (1970) Development, structure and composition of lampbrush chromosomes in domestic fowl. Can J Genet Cytol 12:728–737. https://doi.org/10.1139/g70-095
Angelier N, Bonnanfant-Ja’s ML, Moreau N, et al (1986) DNA methylation and RNA transcriptional activity in amphibian lampbrush chromosomes. Chromosoma 94:169–182. https://doi.org/10.1007/BF00288491
Beagan JA, Phillips-Cremins JE (2020) On the existence and functionality of topologically associating domains. Nat Genet 52:8–16. https://doi.org/10.1038/s41588-019-0561-1
Bickmore WA, van Steensel B (2013) Genome architecture: domain organization of interphase chromosomes. Cell 152:1270–1284. https://doi.org/10.1016/j.cell.2013.02.001
Bintu B, Mateo LJ, Su J-H, et al (2018) Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science 362:eaau1783. https://doi.org/10.1126/science.aau1783
Bione S, Sala C, Manzini C et al (1998) A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. The American Journal of Human Genetics 62:533–541. https://doi.org/10.1086/301761
Bouwman BA, de Laat W (2015) Getting the genome in shape: the formation of loops, domains and compartments. Genome Biol 16:154. https://doi.org/10.1186/s13059-015-0730-1
Buchwalter A, Kaneshiro JM, Hetzer MW (2019) Coaching from the sidelines: the nuclear periphery in genome regulation. Nat Rev Genet 20:39–50. https://doi.org/10.1038/s41576-018-0063-5
Burt DW (2002) Origin and evolution of avian microchromosomes. Cytogenet Genome Res 96:97–112. https://doi.org/10.1159/000063018
Callebaut M (1973) Correlation between germinal vesicle and oocyte development in the adult Japanese quail (Coturnix coturnix japonica). A Cytochemical and Autoradiographic Study Development 29:145–157. https://doi.org/10.1242/dev.29.1.145
Carreira-Rosario A, Bhargava V, Hillebrand J et al (2016) Repression of Pumilio protein expression by Rbfox1 promotes germ cell differentiation. Dev Cell 36:562–571. https://doi.org/10.1016/j.devcel.2016.02.010
Chelysheva LA, Solovei IV, Rodionov AV et al (1990) The lampbrush chromosomes of the chicken. Cytological maps of the macrobivalents. Tsitologiia 32:303–316
Conboy JG (2017) Developmental regulation of RNA processing by Rbfox proteins: Rbfox regulation of RNA processing. Wires RNA 8:e1398. https://doi.org/10.1002/wrna.1398
Cremer M, Grasser F, Lanctôt C et al (2012) Multicolor 3D fluorescence in situ hybridization for imaging interphase chromosomes. In: Hancock R (ed) The Nucleus. Humana Press, Totowa, NJ, pp 205–239
Cremer M, Brandstetter K, Maiser A et al (2020) Cohesin depleted cells rebuild functional nuclear compartments after endomitosis. Nat Commun 11:6146. https://doi.org/10.1038/s41467-020-19876-6
Daks AA, Deriusheva SE, Krasikova AV et al (2010) Lampbrush chromosomes of the Japanese quail (Coturnix coturnix japonica): a new version of cytogenetic maps. Genetika 46:1335–1338
Dekker J (2016) Mapping the 3D genome: aiming for consilience. Nat Rev Mol Cell Biol 17:741–742. https://doi.org/10.1038/nrm.2016.151
Derjusheva S, Kurganova A, Krasikova A et al (2003) Precise identification of chicken chromosomes in the lampbrush form using chromosome painting probes. Chromosome Res 11:749–757. https://doi.org/10.1023/B:CHRO.0000005778.72909.4d
Deryusheva S, Krasikova A, Kulikova T, Gaginskaya E (2007) Tandem 41-bp repeats in chicken and Japanese quail genomes: FISH mapping and transcription analysis on lampbrush chromosomes. Chromosoma 116:519–530. https://doi.org/10.1007/s00412-007-0117-5
Dixon JR, Selvaraj S, Yue F et al (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485:376–380. https://doi.org/10.1038/nature11082
Dixon JR, Gorkin DU, Ren B (2016) Chromatin domains: the unit of chromosome organization. Mol Cell 62:668–680. https://doi.org/10.1016/j.molcel.2016.05.018
Du Z, Zheng H, Kawamura YK et al (2020) Polycomb group proteins regulate chromatin architecture in mouse oocytes and early embryos. Mol Cell 77:825-839.e7. https://doi.org/10.1016/j.molcel.2019.11.011
Eagen KP, Hartl TA, Kornberg RD (2015) Stable chromosome condensation revealed by chromosome conformation capture. Cell 163:934–946. https://doi.org/10.1016/j.cell.2015.10.026
Filippova D, Patro R, Duggal G, Kingsford C (2014) Identification of alternative topological domains in chromatin. Algorithms Mol Biol 9:14. https://doi.org/10.1186/1748-7188-9-14
Fishman V, Battulin N, Nuriddinov M et al (2019) 3D organization of chicken genome demonstrates evolutionary conservation of topologically associated domains and highlights unique architecture of erythrocytes’ chromatin. Nucleic Acids Res 47:648–665. https://doi.org/10.1093/nar/gky1103
Flyamer IM, Gassler J, Imakaev M et al (2017) Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature 544:110–114. https://doi.org/10.1038/nature21711
Freshney R (2010) Culture of animal cells: a manual of basic technique and specialized applications. John Wiley & Sons Inc, Hoboken, NJ, USA
Fudenberg G, Imakaev M, Lu C et al (2016) Formation of chromosomal domains by loop extrusion. Cell Rep 15:2038–2049. https://doi.org/10.1016/j.celrep.2016.04.085
Gaginskaya E, Kulikova T, Krasikova A (2009) Avian lampbrush chromosomes: a powerful tool for exploration of genome expression. Cytogenet Genome Res 124:251–267. https://doi.org/10.1159/000218130
Galkina S, Deryusheva S, Fillon V et al (2006) FISH on avian lampbrush chromosomes produces higher resolution gene mapping. Genetica 128:241–251. https://doi.org/10.1007/s10709-005-5776-7
Gall JG, Callan HG (1962) H3 uridine incorporation in lampbrush chromosomes. Proc Natl Acad Sci 48:562–570. https://doi.org/10.1073/pnas.48.4.562
Gardner EJ, Nizami ZF, Talbot CC Jr, Gall JG (2012) Stable intronic sequence RNA (sisRNA), a new class of noncoding RNA from the oocyte nucleus of Xenopus tropicalis. Genes Dev 15;26(22):2550–9. https://doi.org/10.1101/gad.202184.112
Gibcus JH, Dekker J (2013) The hierarchy of the 3D genome. Mol Cell 49:773–782. https://doi.org/10.1016/j.molcel.2013.02.011
Giorgetti L, Heard E (2016) Closing the loop: 3C versus DNA FISH. Genome Biol 17:215. https://doi.org/10.1186/s13059-016-1081-2
Goetze S, Mateos-Langerak J, van Driel R (2007) Three-dimensional genome organization in interphase and its relation to genome function. Semin Cell Dev Biol 18:707–714. https://doi.org/10.1016/j.semcdb.2007.08.007
Griffin DK, Robertson LBW, Tempest HG, Skinner BM (2007) The evolution of the avian genome as revealed by comparative molecular cytogenetics. Cytogenet Genome Res 117:64–77. https://doi.org/10.1159/000103166
Hartley SE, Callan HG (1978) RNA transcription on the giant lateral loops of the lampbrush chromosomes of the American newt Notophthalmus viridescens. J Cell Sci 34:279–288. https://doi.org/10.1242/jcs.34.1.279
Heng HHQ, Tsui L-C (1993) Modes of DAPI banding and simultaneous in situ hybridization. Chromosoma 102:325–332. https://doi.org/10.1007/BF00661275
Holwerda S, Laat W de (2012) Chromatin loops, gene positioning, and gene expression. Front Gene 3. https://doi.org/10.3389/fgene.2012.00217
Hori T, Suzuki Y, Solovei I et al (1996) Characterization of DNA sequences constituting the terminal heterochromatin of the chicken Z chromosome. Chromosome Res 4:411–426. https://doi.org/10.1007/BF02265048
Hutchison N (1987) Lampbrush chromosomes of the chicken, Gallus domesticus. J Cell Biol 105:1493–1500. https://doi.org/10.1083/jcb.105.4.1493
Iannuccelli E, Mompart F, Gellin J et al (2010) NEMO: a tool for analyzing gene and chromosome territory distributions from 3D-FISH experiments. Bioinformatics 26:696–697. https://doi.org/10.1093/bioinformatics/btq013
Kaufmann R, Cremer C, Gall JG (2012) Superresolution imaging of transcription units on newt lampbrush chromosomes. Chromosome Res 20:1009–1015. https://doi.org/10.1007/s10577-012-9306-z
Keinath MC, Davidian A, Timoshevskiy V, Timoshevskaya N, Gall JG (2021) Characterization of axolotl lampbrush chromosomes by fluorescence in situ hybridization and immunostaining. Exp Cell Res 401:112523. https://doi.org/10.1016/j.yexcr.2021.112523
Knoll JHM, Lichter P (2005) In situ hybridization to metaphase chromosomes and interphase nuclei. Current Protocols in Human Genetics 45. https://doi.org/10.1002/0471142905.hg0403s45
Kolesnikova TD, Goncharov FP, Zhimulev IF (2018) Similarity in replication timing between polytene and diploid cells is associated with the organization of the Drosophila genome. PLoS ONE 13:e0195207. https://doi.org/10.1371/journal.pone.0195207
Krasikova AV, Kulikova TV (2017) Distribution of heterochromatin markers in lampbrush chromosomes in birds. Russ J Genet 53:1022–1029. https://doi.org/10.1134/S1022795417090071
Krasikova A, Deryusheva S, Galkina S et al (2006) On the positions of centromeres in chicken lampbrush chromosomes. Chromosome Res 14:777–789. https://doi.org/10.1007/s10577-006-1085-y
Krasikova A, Daks A, Zlotina A, Gaginskaya E (2009) Polymorphic heterochromatic segments in Japanese quail microchromosomes. Cytogenet Genome Res 126:148–155. https://doi.org/10.1159/000245914
Krasikova A, Fukagawa T, Zlotina A (2012) High-resolution mapping and transcriptional activity analysis of chicken centromere sequences on giant lampbrush chromosomes. Chromosome Res 20:995–1008. https://doi.org/10.1007/s10577-012-9321-0
Kropotova EVGER (1984) Lampbrush chromosomes from the Japanese quail oocytes. Tsitologiia 26:1008–1015
Kulikova T, Chervyakova D, Zlotina A et al (2016) Giant poly(A)-rich RNP aggregates form at terminal regions of avian lampbrush chromosomes. Chromosoma 125:709–724. https://doi.org/10.1007/s00412-015-0563-4
Kulikova T, Surkova A, Zlotina A, Krasikova A (2020) Mapping epigenetic modifications on chicken lampbrush chromosomes. Mol Cytogenet 13:32. https://doi.org/10.1186/s13039-020-00496-0
Lupiáñez DG, Kraft K, Heinrich V et al (2015) Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161:1012–1025. https://doi.org/10.1016/j.cell.2015.04.004
Ma J, Fukuda Y, Schultz RM (2015) Mobilization of dormant Cnot7 mRNA promotes deadenylation of maternal transcripts during mouse oocyte maturation1. Biology of Reproduction 93. https://doi.org/10.1095/biolreprod.115.130344
Macgregor HC (2012) Chromomeres revisited. Chromosome Res 20:911–924. https://doi.org/10.1007/s10577-012-9310-3
Malewska A, Olszańska B (1999) Accumulation and localisation of maternal RNA in oocytes of Japanese quail. Zygote 7:51–59. https://doi.org/10.1017/S0967199499000398
Mandon-Pépin B, Oustry-Vaiman A, Vigier B et al (2003) Expression profiles and chromosomal localization of genes controlling meiosis and follicular development in the sheep ovary. Biol Reprod 68:985–995. https://doi.org/10.1095/biolreprod.102.008557
Maslova A, Krasikova A (2021) FISH going meso-scale: a microscopic search for chromatin domains. Front Cell Dev Biol 9:753097. https://doi.org/10.3389/fcell.2021.753097
Mehlmann LM (2013) Signaling for meiotic resumption in granulosa cells, cumulus cells, and oocyte. In: Coticchio G, Albertini DF, De Santis L (eds) Oogenesis. Springer, London, pp 171–182
Mirny LA, Solovei I (2021) Keeping chromatin in the loop(s). Nat Rev Mol Cell Biol 22:439–440. https://doi.org/10.1038/s41580-021-00337-x
Mishima Y, Tomari Y (2017) Pervasive yet nonuniform contributions of Dcp2 and Cnot7 to maternal mRNA clearance in zebrafish. Genes Cells 22:670–678. https://doi.org/10.1111/gtc.12504
Misteli T (2020) The self-organizing genome: principles of genome architecture and function. Cell 183:28–45. https://doi.org/10.1016/j.cell.2020.09.014
Morgan GT (2018) Imaging the dynamics of transcription loops in living chromosomes. Chromosoma 127:361–374. https://doi.org/10.1007/s00412-018-0667-8
Morgan GT, Jones P, Bellini M (2012) Association of modified cytosines and the methylated DNA-binding protein MeCP2 with distinctive structural domains of lampbrush chromatin. Chromosome Res 20:925–942. https://doi.org/10.1007/s10577-012-9324-x
Nora EP, Lajoie BR, Schulz EG et al (2012) Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 485:381–385. https://doi.org/10.1038/nature11049
Razin SV, Gavrilov AA, Vassetzky YS, Ulianov SV (2016) Topologically-associating domains: gene warehouses adapted to serve transcriptional regulation. Transcription 7:84–90. https://doi.org/10.1080/21541264.2016.1181489
Robinson JT, Turner D, Durand NC et al (2018) Juicebox.js provides a cloud-based visualization system for Hi-C data. Cell Syst 6:256-258.e1. https://doi.org/10.1016/j.cels.2018.01.001
Rowley MJ, Nichols MH, Lyu X et al (2017) Evolutionarily conserved principles predict 3D chromatin organization. Mol Cell 67:837-852.e7. https://doi.org/10.1016/j.molcel.2017.07.022
Shibusawa M, Nishibori M, Nishida-Umehara C et al (2004) Karyotypic evolution in the Galliformes: an examination of the process of karyotypic evolution by comparison of the molecular cytogenetic findings with the molecular phylogeny. Cytogenet Genome Res 106:111–119. https://doi.org/10.1159/000078570
Simeoni I, Gilchrist MJ, Garrett N, Armisen J, Gurdon JB (2012) Widespread transcription in an amphibian oocyte relates to its reprogramming activity on transplanted somatic nuclei. Stem Cells and Development 21(2):181–190. https://doi.org/10.1089/scd.2011.0162
Solovei I, Gaginskaya E, Allen T, Macgregor H (1992) A novel structure associated with a lampbrush chromosome in the chicken, Gallus domesticus. J Cell Sci 101:759–772. https://doi.org/10.1242/jcs.101.4.759
Solovei I, Gaginskaya ER, Macgregor HC (1994) The arrangement and transcription of telomere DNA sequences at the ends of lampbrush chromosomes of birds. Chromosome Res 2:460–470. https://doi.org/10.1007/BF01552869
Sun Y-L, Zeng S, Ye K et al (2012) Involvement of FGF9/16/20 subfamily in female germ cell development of the Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem 38:1427–1439. https://doi.org/10.1007/s10695-012-9630-4
Symmons O, Pan L, Remeseiro S et al (2016) The Shh topological domain facilitates the action of remote enhancers by reducing the effects of genomic distances. Dev Cell 39:529–543. https://doi.org/10.1016/j.devcel.2016.10.015
Szabo Q, Donjon A, Jerković I et al (2020) Regulation of single-cell genome organization into TADs and chromatin nanodomains. Nat Genet 52:1151–1157. https://doi.org/10.1038/s41588-020-00716-8
Trzaskoma P, Ruszczycki B, Lee B et al (2020) Ultrastructural visualization of 3D chromatin folding using volume electron microscopy and DNA in situ hybridization. Nat Commun 11:2120. https://doi.org/10.1038/s41467-020-15987-2
Ulianov SV, Khrameeva EE, Gavrilov AA et al (2016) Active chromatin and transcription play a key role in chromosome partitioning into topologically associating domains. Genome Res 26:70–84. https://doi.org/10.1101/gr.196006.115
Virnicchi G, Bora P, Gahurova L et al (2020) Wwc2 is a novel cell division regulator during preimplantation mouse embryo lineage formation and oogenesis. Front Cell Dev Biol 8:857. https://doi.org/10.3389/fcell.2020.00857
Vlad M, Macgregor HC (1975) Chromomere number and its genetic significance in lampbrush chromosomes. Chromosoma 50:327–347. https://doi.org/10.1007/BF00327073
Weber T, Schmidt E, Scheer U (1989) Mapping of transcription units on Xenopus laevis lampbrush chromosomes by in situ hybridization with biotin-labeled cDNA probes. Eur J Cell Biol 50:144–153
Weinreb C, Raphael BJ (2016) Identification of hierarchical chromatin domains. Bioinformatics 32:1601–1609. https://doi.org/10.1093/bioinformatics/btv485
Yang X, Zhao F, Han Q, et al (2020) Transcriptome analysis in the shell gland of laying hens affecting eggshell qualities. In Review
Zhu Z, Miao Z, Chen H et al (2016) Ovarian transcriptomic analysis of Shan Ma ducks at peak and late stages of egg production. Asian-Australas J Anim Sci 30:1215–1224. https://doi.org/10.5713/ajas.16.0470
Zlotina A, Krasikova A (2017) FISH in lampbrush chromosomes. In: Liehr T (ed) Fluorescence in situ hybridization (FISH). Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 445–457
Zlotina A, Galkina S, Krasikova A et al (2012) Centromere positions in chicken and Japanese quail chromosomes: de novo centromere formation versus pericentric inversions. Chromosome Res 20:1017–1032. https://doi.org/10.1007/s10577-012-9319-7
Zlotina A, Kulikova T, Kosyakova N et al (2016) Microdissection of lampbrush chromosomes as an approach for generation of locus-specific FISH-probes and samples for high-throughput sequencing. BMC Genomics 17:126. https://doi.org/10.1186/s12864-016-2437-4
Zlotina A, Maslova A, Pavlova O et al (2020) New insights into chromomere organization provided by lampbrush chromosome microdissection and high-throughput sequencing. Front Genet 11:57. https://doi.org/10.3389/fgene.2020.00057
Funding
The research was supported by the Russian Science Foundation (grant #19–74-20075) and was performed using the equipment of the Resource Center “Molecular and Cell Technologies” (Saint Petersburg State University).
Author information
Authors and Affiliations
Contributions
AK conceived the study and supervised the project. AM performed 3D-FISH on chicken embryonic fibroblasts, image analysis, and 3D-measurements. TK, PS, and SR performed 2D-FISH on isolated lampbrush chromosomes. TK, AK, and SR performed bioinformatic analysis of the mapped genomic sequences. TK, AM, and AK drafted the manuscript. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kulikova, T., Maslova, A., Starshova, P. et al. Comparison of the somatic TADs and lampbrush chromomere-loop complexes in transcriptionally active prophase I oocytes. Chromosoma 131, 207–223 (2022). https://doi.org/10.1007/s00412-022-00780-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-022-00780-5