Abstract
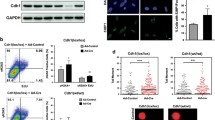
Replication stress often induces chromosome instability. In this study, we explore which factors in replication-compromised cells promote abnormal chromosome ploidy. We expressed mutant forms of either polymerase α (Polα) or polymerase δ (Polδ) in normal human fibroblasts to compromise DNA replication. Cells expressing the mutant Polα-protein failed to sustain mitotic arrest and, when propagated progressively, down-regulated Mad2 and BubR1 and accumulated 4N-DNA from the 2N-DNA cells. Significantly, a population of these cells became tetraploids. The Polα mutant expressing cells also exhibited elevated cellular senescence markers, suggesting as a mechanism to limit proliferation of the tetraploids. Expression of the Polδ mutant also caused cells to accumulate 4N-DNA. In contrast to the Polα mutant expressing cells, the Polδ mutant expressing cells expressed sufficient levels of Mad2, BubR1, and cyclin B1 to sustain mitotic arrest, and these cells had normal chromosome ploidy. Together, these results suggest that replication-compromised cells depend on the mitotic checkpoint to prevent mitotic slippage that could result in tetraploidization.






Similar content being viewed by others
References
Andreassen PR, Lohez OD, Lacroix FB, Margolis RL (2001) Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol Biol Cell 12:1315–1328
Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71:333–374
Brito DA, Rieder CL (2006) Mitotic checkpoint slippage in humans occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol 16:1194–1200
Campisi J (2001) Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol 11:S27–S31
Campisi J, d'Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8:729–740
Chan KL, Palmai-Pallag T, Ying S, Hickson ID (2009) Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat Cell Biol 11:753–760
Dahlen M, Sunnerhagen P, Wang TS (2003) Replication proteins influence the maintenance of telomere length and telomerase protein stability. Mol Cell Biol 23:3031–3042
Dai W, Wang Q, Liu T, Swamy M, Fang Y, Xie S, Mahmood R, Yang YM, Xu M, Rao CV (2004) Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res 64:440–445
Duelli D, Lazebnik Y (2007) Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer 7:968–976
Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D (2005) Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437:1043–1047
Ganem NJ, Storchova Z, Pellman D (2007) Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev 17:157–162
Gutierrez PJ, Wang TS-F (2003) Genomic instability induced by mutations in Saccharomyces cerevisiae POL1. Genetics 165:65–81
Kortlever RM, Higgins PJ, Bernards R (2006) Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 8:877–884
Michel LS, Liberal V, Chatterjee A, Kirchwegger R, Pasche B, Gerald W, Dobles M, Sorger PK, Murty VV, Benezra R (2001) MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature 409:355–359
Miles J, Formosa T (1992) Protein affinity chromatography with purified yeast DNA polymerase alpha detects proteins that bind to DNA polymerase. Proc Natl Acad Sci USA 89:1276–1280
Narita M, Lowe SW (2005) Senescence comes of age. Nat Med 11:920–922
Narita M, Nunez S, Heard E, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW (2003) Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113:703–716
Pellman D (2007) Cell biology: aneuploidy and cancer. Nature 446:38–39
Prasanth SG, Prasanth KV, Stillman B (2002) Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science 297:1026–1031
Rajagopalan H, Lengauer C (2004a) Aneuploidy and cancer. Nature 432:338–341
Rajagopalan H, Lengauer C (2004b) CIN-ful cancers. Cancer Chemother Pharmacol 54(Suppl 1):S65–S568
Rieder CL, Maiato H (2004) Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell 7:637–651
Rodier F, Campisi J (2009) When DNA damage goes invisible. Cell Cycle 8:3632–3633
Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11:973–979
Shi Q, King RW (2005) Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437:1038–1042
Shin HJ, Baek KH, Jeon AH, Park MT, Lee SJ, Kang CM, Lee HS, Yoo SH, Chung DH, Sung YC et al (2003) Dual roles of human BubR1, a mitotic checkpoint kinase, in the monitoring of chromosomal instability. Cancer Cell 4:483–497
Stillman B (2008) DNA polymerases at the replication fork in eukaryotes. Mol Cell 30:259–260
Storchova Z, Kuffer C (2008) The consequences of tetraploidy and aneuploidy. J Cell Sci 121:3859–3866
Storchova Z, Pellman D (2004) From polyploidy to aneuploidy, genome instability and cancer. Nat Rev Mol Cell Biol 5:45–54
Tanaka H, Kubota Y, Tsujimura T, Kumano M, Masai H, Takisawa H (2009) Replisome progression complex links DNA replication to sister chromatid cohesion in Xenopus egg extracts. Genes Cells 14:949–963
Uetake Y, Sluder G (2004) Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a “tetraploidy checkpoint”. J Cell Biol 165:609–615
Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW (2007) Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11:25–36
Wittmeyer J, Formosa T (1997) The Saccharomyces cerevisiae DNA polymerase alpha catalytic subunit interacts with Cdc68/Spt16 and with Pob3, a protein similar to an HMG1-like protein. Mol Cell Biol 17:4178–4190
Wong C, Stearns T (2005) Mammalian cells lack checkpoints for tetraploidy, aberrant centrosome number, and cytokinesis failure. BMC Cell Biol 6:6
Yaswen P, Campisi J (2007) Oncogene-induced senescence pathways weave an intricate tapestry. Cell 128:233–234
Yoshizawa-Sugata N, Masai H (2009) Roles of human AND-1 in chromosome transactions in S phase. J Biol Chem 284:20718–20728
Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL et al (2005) Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell 8:19–30
Zhou Y, Wang TS-F (2004) A coordinated temporal interplay of nucleosome reorganization factor, sisiter chromatin cohesion factor, and DNA polymerase α facilitates DNA replication. Mol Cell Biol 24:9568–9579
Zhu W, Ukomadu C, Jha S, Senga T, Dhar SK, Wohlschlegel JA, Nutt LK, Kornbluth S, Dutta A (2007) Mcm10 and And-1/CTF4 recruit DNA polymerase alpha to chromatin for initiation of DNA replication. Genes Dev 21:2288–2299
Zimmet J, Ravid K (2000) Polyploidy: occurrence in nature, mechanisms, and significance for the megakaryocyte–platelet system. Exp Hematol 28:3–16
Acknowledgments
We thank Dr. Guowei Fang of Stanford University for antibodies against Mad2 and BubR1. This work is supported by grants from National Cancer Institute of National Institutes of Health. Z. Z. is partially supported by the Dean’s Fellowship of Stanford University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Diffley
Rights and permissions
About this article
Cite this article
Zhang, Z., Arora, S., Zhou, Y. et al. Replication-compromised cells require the mitotic checkpoint to prevent tetraploidization. Chromosoma 120, 73–82 (2011). https://doi.org/10.1007/s00412-010-0292-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-010-0292-7