Abstract
Centromeric protein F (CENP-F) is a 367-kDa human kinetochore protein that was identified a decade ago, but its function was only recently revealed by studies that used small interfering RNA to deplete the protein from cells. All studies showed that CENP-F is important for chromosome alignment, but these studies differed as to whether CENP-F is important to the mitotic checkpoint. We report here that CENP-F is essential for cells to sustain a prolonged mitotic delay in response to unattached kinetochores. Cells depleted of CENP-F exit mitosis in the presence of defective kinetochore attachments resulting from treatment with nocodazole, or the depletion of kinetochore proteins CENP-E and hSgo1. Kinetochores depleted of CENP-F exhibited a reduction in the amounts of the mitotic checkpoint proteins Mad1, Mad2, hBUBR1, hBUB1, and hMps1. We postulate that CENP-F is not an essential component of the mitotic checkpoint but facilitates the duration of the mitotic delay. Separately, we show that CENP-F is a novel microtubule-binding protein that possesses two microtubule-binding domains at opposite ends of the molecule. The C-terminal microtubule-binding domain was found to stimulate microtubule polymerization in vitro. These activities provide a biochemical explanation for how CENP-F contributes to kinetochore attachments in vivo.







Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Ashar HR, James L, Gray K, Carr D, Black S, Armstrong L, Bishop WR, Kirschmeier P (2000) Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem 275:30451–30457
Bomont P, Maddox P, Shah JV, Desai AB, Cleveland DW (2005) Unstable microtubule capture at kinetochores depleted of the centromere-associated protein CENP-F. EMBO J 24:3927–3939
Casiano CA, Landberg G, Ochs RL, Tan EM (1993) Autoantibodies to a novel cell cycle-regulated protein that accumulates in the nuclear matrix during S phase and is localized in the kinetochores and spindle midzone during mitosis. J Cell Sci 106(4):1045–1056
Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB (1996) Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol 132:617–633
Hoffman DB, Pearson CG, Yen TJ, Howell BJ, Salmon ED (2001) Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol Biol Cell 12:1995–2009
Holt SV, Vergnolle MA, Hussein D, Wozniak MJ, Allan VJ, Taylor SS (2005) Silencing Cenp-F weakens centromeric cohesion, prevents chromosome alignment and activates the spindle checkpoint. J Cell Sci 118:4889–900
Hussein D, Taylor SS (2002) Farnesylation of Cenp-F is required for G2/M progression and degradation after mitosis. J Cell Sci 115:3403–3414
Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS (2004) Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci 117:1577–1589
Joseph J, Liu ST, Jablonski SA, Yen TJ, Dasso M (2004) The RanGAP1–RanBP2 complex is essential for microtubule–kinetochore interactions in vivo. Curr Biol 14:611–617
Laoukili J, Kooistra MR, Bras A, Kauw J, Kerkhoven RM, Morrison A, Clevers H, Medema RH (2005) FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol 7:126–136
Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ (1995) CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol 130:507–518
Liu ST, Hittle JC, Jablonski SA, Campbell MS, Yoda K, Yen TJ (2003) Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nat Cell Biol 5:341–345
McEwen BF, Chan GK, Zubrowski B, Savoian MS, Sauer MT, Yen TJ (2001) CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol Biol Cell 12:2776–2789
Rattner JB, Rao A, Fritzler MJ, Valencia DW, Yen TJ (1993) CENP-F is a .ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil Cytoskeleton 26:214–226
Salina D, Enarson P, Rattner JB, Burke B (2003) Nup358 integrates nuclear envelope breakdown with kinetochore assembly. J Cell Biol 162:991–1001
Steensgaard P, Garre M, Muradore I, Transidico P, Nigg EA, Kitagawa K, Earnshaw WC, Faretta M, Musacchio A (2004) Sgt1 is required for human kinetochore assembly. EMBO Rep 5:626–631
Yang Z, Guo J, Chen Q, Ding C, Du J, Zhu X (2005) Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol Cell Biol 25:4062–4074
Zhu X, Chang KH, He D, Mancini MA, Brinkley WR, Lee WH (1995a) The C terminus of mitosin is essential for its nuclear localization, centromere/kinetochore targeting, and dimerization. J Biol Chem 270:19545–19550
Zhu X, Mancini M, Chang K, Liu C, Chen C, Shan B, Jones D, Yang-Feng T, Lee W (1995b) Characterization of a novel 350-kilodalton nuclear phosphoprotein that is specifically involved in mitotic-phase progression. Mol Cell Biol 15:5017–5029
Acknowledgements
We acknowledge the excellent technical support by J. Hittle and B. Conner, and advice from S.T. Liu and other members of the Yen lab. We give special thanks to J. Peterson for the use of equipment. This work is supported by the National Institute of Health GM44762, core grants CA06927 CA75138, and an appropriation from the Commonwealth of Pennsylvania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E.A. Nigg
Electronic supplementary material
Below is the image and a link to the electronic supplementary material
Fig8
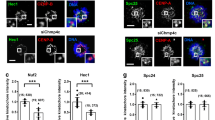
CENP-F is not essential for checkpoint proteins to assemble onto kinetochores during prometaphase. Hela cells transfected with control and CENP-F siRNAs were fixed and stained for CENP-F and Bub1, BubR1, MPS1, Mad1 or Mad2. Scale bar: 10 μm. Cells in prometaphase were selected and only those whose kinetochores were depleted of CENP-F by >25-fold were chosen for analysis. The strongest signal detected at a control kinetochore was chosen as 100%. Approximately 150 kinetochores were quantified for each antibody. Error bars represent s.e.m
Rights and permissions
About this article
Cite this article
Feng, J., Huang, H. & Yen, T.J. CENP-F is a novel microtubule-binding protein that is essential for kinetochore attachments and affects the duration of the mitotic checkpoint delay. Chromosoma 115, 320–329 (2006). https://doi.org/10.1007/s00412-006-0049-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-006-0049-5