Abstract
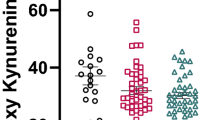
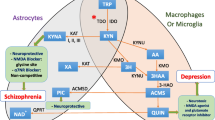
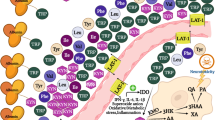
Schizophrenia is a chronic psychotic disease burdened by cognitive deficits which hamper daily functioning causing disability and costs for society. Biological determinants underlying cognitive impairment are only partially understood and there are no convincing pharmacological targets able to improve cognitive outcome. Mounting evidence has shown the involvement of the kynurenine pathway in the pathophysiology of schizophrenia, also concerning cognitive symptoms. Therefore, the action of specific metabolites of kynurenine could affects cognition in schizophrenia. To evaluate the impact of the metabolites of kynurenine pathway on cognitive functions in schizophrenia spectrum disorders, with a focus on the modulating role of gender, to identify predictors of cognitive functioning and hypothetical pharmacological targets able to resize disability by improving cognition, thus functioning and quality of life. A systematic review was performed in PubMed/MEDLINE and Embase according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses. All studies measuring the direct impact of kynurenine metabolites on cognitive performances in living individuals with schizophrenia spectrum disorders were included in the review. Six studies were included. The activation of the kynurenine pathway resulted associated with greater cognitive deficits in patients with schizophrenia and both elevations and reduction of metabolites seemed able to affect cognitive outcome. No modulating role of sex emerged. This systematic review provides evidence that the activation of the kynurenine pathway affects cognition in patients with schizophrenia and highlights this pathway as a possible future target for developing novel drugs toward this still unmet clinical need. However, evidence is still limited and future studies are needed to further clarify the relationship between kynurenine pathway and cognition in schizophrenia.


Similar content being viewed by others
Data availability
No datasets were analyzed or generated during the course of the current study.
References
Tandon R, Nasrallah HA, Keshavan MS (2009) Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res 110:1–23. https://doi.org/10.1016/j.schres.2009.03.005
Bechi M, Bosia M, Spangaro M et al (2017) Exploring functioning in schizophrenia: predictors of functional capacity and real-world behaviour. Psychiatry Res 251:118–124. https://doi.org/10.1016/J.PSYCHRES.2017.02.019
Gebreegziabhere Y, Habatmu K, Mihretu A et al (2022) Cognitive impairment in people with schizophrenia: an umbrella review. Eur Arch Psychiatry Clin Neurosci 272:1139–1155. https://doi.org/10.1007/s00406-022-01416-6
Wonodi I, Schwarcz R (2010) Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in schizophrenia. Schizophr Bull 36:211–218. https://doi.org/10.1093/SCHBUL/SBQ002
Krogmann A, Peters L, Von Hardenberg L et al (2019) Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr 24:41–68. https://doi.org/10.1017/S109285291900124X
Bosia M, Spangaro M, Sapienza J et al (2021) Cognition in schizophrenia: modeling the interplay between interleukin-1β C-511T polymorphism, metabolic syndrome, and sex. Neuropsychobiology 80:321–332. https://doi.org/10.1159/000512082
Zhang B, Han M, Tan S et al (2017) Gender differences measured by the MATRICS consensus cognitive battery in chronic schizophrenia patients. Sci Rep 7:11821. https://doi.org/10.1038/S41598-017-12027-W
D. Howes O, Fusar-Poli P, Bloomfield M, et al (2012) From the prodrome to chronic schizophrenia: the neurobiology underlying psychotic symptoms and cognitive impairments. Curr Pharm Des 18:459–465. https://doi.org/10.2174/138161212799316217
Howes OD, Kapur S (2009) The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull 35:549–562. https://doi.org/10.1093/schbul/sbp006
Braver TS, Barch DM, Cohen JD (1999) Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry 46:312–328. https://doi.org/10.1016/S0006-3223(99)00116-X
Bosia M, Bechi M, Pirovano A et al (2014) COMT and 5-HT1A-receptor genotypes potentially affect executive functions improvement after cognitive remediation in schizophrenia. Heal Psychol Behav Med 2:509–516. https://doi.org/10.1080/21642850.2014.905206
Bosia M, Pigoni A, Pirovano A et al (2015) COMT and STH polymorphisms interaction on cognition in schizophrenia. Neurol Sci 36:215–220. https://doi.org/10.1007/S10072-014-1936-9
Bosia M, Bechi M, Marino E et al (2007) Influence of catechol-O-methyltransferase Val158Met polymorphism on neuropsychological and functional outcomes of classical rehabilitation and cognitive remediation in schizophrenia. Neurosci Lett 417:271–274. https://doi.org/10.1016/j.neulet.2007.02.076
He Q, Xue G, Chen C et al (2012) COMT Val158Met polymorphism interacts with stressful life events and parental warmth to influence decision making. Sci Rep 2:677. https://doi.org/10.1038/SREP00677
Koola MM (2021) Alpha7 nicotinic-N-methyl-d-aspartate hypothesis in the treatment of schizophrenia and beyond. Hum Psychopharmacol 36:1–16. https://doi.org/10.1002/HUP.2758
Spangaro M, Bosia M, Zanoletti A et al (2012) Cognitive dysfunction and glutamate reuptake: effect of EAAT2 polymorphism in schizophrenia. Neurosci Lett 522:151–155. https://doi.org/10.1016/J.NEULET.2012.06.030
Spangaro M, Bosia M, Zanoletti A et al (2014) Exploring effects of EAAT polymorphisms on cognitive functions in schizophrenia. Pharmacogenomics 15:925–932. https://doi.org/10.2217/PGS.14.42
Khandaker GM, Cousins L, Deakin J et al (2015) Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2:258–270. https://doi.org/10.1016/S2215-0366(14)00122-9
Kogan S, Ospina LH, Mittal VA, Kimhy D (2019) The impact of inflammation on neurocognition and risk for psychosis: a critical review. Eur Arch Psychiatry Clin Neurosci 270:793–802. https://doi.org/10.1007/s00406-019-01073-2
Yamada S, Takahashi S, Malchow B et al (2022) Cognitive and functional deficits are associated with white matter abnormalities in two independent cohorts of patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 272:957–969. https://doi.org/10.1007/s00406-021-01363-8
Na KS, Jung HY, Kim YK (2014) The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 48:277–286. https://doi.org/10.1016/j.pnpbp.2012.10.022
Maas DA, Vallès A, Martens GJM (2017) Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl Psychiatry 7:e1171. https://doi.org/10.1038/TP.2017.138
Smirnova LP, Yarnykh VL, Parshukova DA et al (2021) Global hypomyelination of the brain white and gray matter in schizophrenia: quantitative imaging using macromolecular proton fraction. Transl Psychiatry 11:365. https://doi.org/10.1038/s41398-021-01475-8
Noto MN, Maes M, Nunes SOV et al (2019) Activation of the immune-inflammatory response system and the compensatory immune-regulatory system in antipsychotic naive first episode psychosis. Eur Neuropsychopharmacol 29:416–431. https://doi.org/10.1016/j.euroneuro.2018.12.008
Comai S, Bertazzo A, Brughera M, Crotti S (2020) Tryptophan in health and disease. Adv Clin Chem 95:165–218. https://doi.org/10.1016/BS.ACC.2019.08.005
Comai S, Melloni E, Lorenzi C et al (2022) Selective association of cytokine levels and kynurenine/tryptophan ratio with alterations in white matter microstructure in bipolar but not in unipolar depression. Eur Neuropsychopharmacol 55:96–109. https://doi.org/10.1016/J.EURONEURO.2021.11.003
Pires AS, Sundaram G, Heng B et al (2021) Recent advances in clinical trials targeting the kynurenine pathway. Pharmacol Ther 236:108055. https://doi.org/10.1016/J.PHARMTHERA.2021.108055
Kindler J, Lim CK, Weickert CS et al (2020) Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry 25:2860–2872. https://doi.org/10.1038/S41380-019-0401-9
de Bie J, Lim CK, Guillemin GJ (2016) Kynurenines, gender and neuroinflammation; showcase schizophrenia. Neurotox Res 30:285–294. https://doi.org/10.1007/S12640-016-9641-5
Schwarcz R, Stone TW (2017) The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology 112:237–247. https://doi.org/10.1016/J.NEUROPHARM.2016.08.003
Comai S, Bertazzo A, Carretti N et al (2010) Serum levels of tryptophan, 5-hydroxytryptophan and serotonin in patients affected with different forms of amenorrhea. Int J Tryptophan Res 3:69–75. https://doi.org/10.4137/IJTR.S3804
Carretti N, Florio P, Bertolin A et al (2005) Serum fluctuations of total and free tryptophan levels during the menstrual cycle are related to gonadotrophins and reflect brain serotonin utilization. Hum Reprod 20:1548–1553. https://doi.org/10.1093/HUMREP/DEH795
Carretti N, Florio P, Reis FM et al (2007) Menopause alters the metabolism of serum serotonin precursors and their correlation with gonadotropins and estradiol. Climacteric 10:393–399. https://doi.org/10.1080/13697130701378198
De Bie J, Guest J, Guillemin GJ, Grant R (2016) Central kynurenine pathway shift with age in women. J Neurochem 136:995–1003. https://doi.org/10.1111/JNC.13496
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13:465–477. https://doi.org/10.1038/NRN3257
Roomruangwong C, Noto C, Kanchanatawan B et al (2020) The role of aberrations in the immune-inflammatory response system (IRS) and the compensatory immune-regulatory reflex system (CIRS) in different phenotypes of schizophrenia: the IRS-CIRS theory of schizophrenia. Mol Neurobiol 57:778–797. https://doi.org/10.1007/S12035-019-01737-Z
Comai S, Costa CVL, Ragazzi E et al (2005) The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clin Chim Acta 360:67–80. https://doi.org/10.1016/J.CCCN.2005.04.013
Fujigaki H, Mouri A, Yamamoto Y et al (2019) Linking phencyclidine intoxication to the tryptophan–kynurenine pathway: therapeutic implications for schizophrenia. Neurochem Int 125:1–6. https://doi.org/10.1016/j.neuint.2019.02.001
Hilmas C, Pereira EFR, Alkondon M et al (2001) The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 21:7463–7473. https://doi.org/10.1523/JNEUROSCI.21-19-07463.2001
Gibbons A, Dean B (2016) The cholinergic system: an emerging drug target for schizophrenia. Curr Pharm Des 22:2124–2133. https://doi.org/10.2174/1381612822666160127114010
Bai MY, Lovejoy DB, Guillemin GJ et al (2021) Galantamine–memantine combination and kynurenine pathway enzyme inhibitors in the treatment of neuropsychiatric disorders. Complex psychiatry 7:19–33. https://doi.org/10.1159/000515066
Anderson G, Maes M (2013) Schizophrenia: linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog Neuro-Psychopharmacol Biol Psychiatry 42:5–19. https://doi.org/10.1016/j.pnpbp.2012.06.014
Upthegrove R, Khandaker GM (2020) Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr Top Behav Neurosci 44:49–66. https://doi.org/10.1007/7854_2018_88
Flatow J, Buckley P, Miller BJ (2013) Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74:400–409. https://doi.org/10.1016/j.biopsych.2013.03.018
Howes OD, McCutcheon R (2017) Inflammation and the neural diathesis–stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry 7:e1024–e1111. https://doi.org/10.1038/tp.2016.278
Miller BJ, Buckley P, Seabolt W et al (2011) Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 70:663–671. https://doi.org/10.1016/j.biopsych.2011.04.013
Momtazmanesh S, Zare-Shahabadi A, Rezaei N (2019) Cytokine alterations in schizophrenia: an updated review. Front Psychiatry 10:892. https://doi.org/10.3389/FPSYT.2019.00892
Chiappelli J, Notarangelo FM, Pocivavsek A et al (2018) Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacology 43:1675–1680. https://doi.org/10.1038/S41386-018-0038-4
Savitz J (2020) The kynurenine pathway: a finger in every pie. Mol Psychiatry 25:131–147. https://doi.org/10.1038/S41380-019-0414-4
Pocivavsek A, Thomas MAR, Elmer GI et al (2014) Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology 231:2799–2809. https://doi.org/10.1007/S00213-014-3452-2
Pershing ML, Bortz DM, Pocivavsek A et al (2015) Elevated levels of kynurenic acid during gestation produce neurochemical, morphological, and cognitive deficits in adulthood: implications for schizophrenia. Neuropharmacology 90:33–41. https://doi.org/10.1016/J.NEUROPHARM.2014.10.017
Erhardt S, Pocivavsek A, Repici M et al (2017) Adaptive and behavioral changes in kynurenine 3-monooxygenase knockout mice: relevance to psychotic disorders. Biol Psychiatry 82:756–765. https://doi.org/10.1016/J.BIOPSYCH.2016.12.011
Almulla AF, Vasupanrajit A, Tunvirachaisakul C et al (2022) The tryptophan catabolite or kynurenine pathway in schizophrenia: meta-analysis reveals dissociations between central, serum, and plasma compartments. Mol Psychiatry. https://doi.org/10.1038/S41380-022-01552-4
Kanchanatawan B, Hemrungrojn S, Thika S et al (2018) Changes in tryptophan catabolite (TRYCAT) pathway patterning are associated with mild impairments in declarative memory in schizophrenia and deficits in semantic and episodic memory coupled with increased false-memory creation in deficit schizophrenia. Mol Neurobiol 55:5184–5201. https://doi.org/10.1007/S12035-017-0751-8
Kirkpatrick B, Buchanan RW, McKenny PD et al (1989) The schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res 30:119–123. https://doi.org/10.1016/0165-1781(89)90153-4
Kirkpatrick B, Galderisi S (2008) Deficit schizophrenia: an update. World Psychiatry 7:143–147. https://doi.org/10.1002/J.2051-5545.2008.TB00181.X
Bechi M, Spangaro M, Agostoni G et al (2019) Intellectual and cognitive profiles in patients affected by schizophrenia. J Neuropsychol 13:589–602. https://doi.org/10.1111/JNP.12161
Huang X, Ding W, Wu F et al (2020) Increased plasma kynurenic acid levels are associated with impaired attention/vigilance and social cognition in patients with schizophrenia. Neuropsychiatr Dis Treat 16:263–271. https://doi.org/10.2147/NDT.S239763
Huang J, Tong J, Zhang P et al (2021) Effects of neuroactive metabolites of the tryptophan pathway on working memory and cortical thickness in schizophrenia. Transl Psychiatry 11:198. https://doi.org/10.1038/S41398-021-01311-Z
Cathomas F, Guetter K, Seifritz E et al (2021) Quinolinic acid is associated with cognitive deficits in schizophrenia but not major depressive disorder. Sci Rep 11:9992. https://doi.org/10.1038/S41598-021-89335-9
Noyan H, Erdağ E, Tüzün E et al (2021) Association of the kynurenine pathway metabolites with clinical, cognitive features and IL-1β levels in patients with schizophrenia spectrum disorder and their siblings. Schizophr Res 229:27–37. https://doi.org/10.1016/J.SCHRES.2021.01.014
Badawy AAB, Guillemin G (2019) The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: time for appraisal. Int J Tryptophan Res 12:1178646919868978. https://doi.org/10.1177/1178646919868978
Fukui S, Schwarcz R, Rapoport SI et al (1991) Blood–brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 56:2007–2017. https://doi.org/10.1111/J.1471-4159.1991.TB03460.X
Pollak TA, Drndarski S, Stone JM et al (2018) The blood–brain barrier in psychosis. Lancet Psychiatry 5:79–92. https://doi.org/10.1016/S2215-0366(17)30293-6
Fellendorf FT, Manchia M, Squassina A et al (2022) Is poor lithium response in individuals with bipolar disorder associated with increased degradation of tryptophan along the kynurenine pathway? Results of an exploratory study. J Clin Med 11:2517. https://doi.org/10.3390/JCM11092517
Braidy N, Grant R, Brew BJ et al (2009) Effects of kynurenine pathway metabolites on intracellular NAD synthesis and cell death in human primary astrocytes and neurons. Int J Tryptophan Res 2:61–69. https://doi.org/10.4137/IJTR.S2318
Braidy N, Guillemin GJ, Grant R (2011) Effects of kynurenine pathway inhibition on NAD metabolism and cell viability in human primary astrocytes and neurons. Int J Tryptophan Res 4:29–37. https://doi.org/10.4137/IJTR.S7052
Plitman E, Nakajima S, de la Fuente-Sandoval C et al (2014) Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol 24:1591–1605. https://doi.org/10.1016/J.EURONEURO.2014.07.015
Shah P, Plitman E, Iwata Y et al (2020) Glutamatergic neurometabolites and cortical thickness in treatment-resistant schizophrenia: implications for glutamate-mediated excitotoxicity. J Psychiatr Res 124:151–158. https://doi.org/10.1016/J.JPSYCHIRES.2020.02.032
Zhang Y, Catts VS, Sheedy D et al (2016) Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry 6:e982. https://doi.org/10.1038/TP.2016.238
Wu D, Lv P, Li F et al (2019) Association of peripheral cytokine levels with cerebral structural abnormalities in schizophrenia. Brain Res 1724:146463. https://doi.org/10.1016/J.BRAINRES.2019.146463
Leung A, Chue P (2000) Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl 401:3–38. https://doi.org/10.1111/J.0065-1591.2000.0AP25.X
Al-Hakeim HK, Almulla AF, Maes M (2020) The neuroimmune and neurotoxic fingerprint of major neurocognitive psychosis or deficit schizophrenia: a supervised machine learning study. Neurotox Res 37:753–771. https://doi.org/10.1007/s12640-019-00112-z
Al-Hakeim HK, Almulla AF, Al-Dujaili AH, Maes M (2020) Construction of a neuro-immune-cognitive pathway-phenotype underpinning the phenome of deficit schizophrenia. Curr Top Med Chem 20:747–758. https://doi.org/10.2174/1568026620666200128143948
Maes M, Vojdani A, Sirivichayakul S et al (2021) Inflammatory and oxidative pathways are new drug targets in multiple episode schizophrenia and leaky gut, Klebsiella pneumoniae, and C1q immune complexes are additional drug targets in first episode schizophrenia. Mol Neurobiol 58:3319–3334. https://doi.org/10.1007/s12035-021-02343-8
Al-Hakeim HK, Mousa RF, Al-Dujaili AH, Maes M (2021) In schizophrenia, non-remitters and partial remitters to treatment with antipsychotics are qualitatively distinct classes with respect to neurocognitive deficits and neuro-immune biomarkers: results of soft independent modeling of class analogy. Metab Brain Dis 36:939–955. https://doi.org/10.1007/S11011-021-00685-9
Maes M, Kanchanatawan B (2021) In (deficit) schizophrenia, a general cognitive decline partly mediates the effects of neuro-immune and neuro-oxidative toxicity on the symptomatome and quality of life. CNS Spectr 12:1–10. https://doi.org/10.1017/S1092852921000419
Lim K, Smucny J, Barch DM et al (2021) Cognitive subtyping in schizophrenia: a latent profile analysis. Schizophr Bull 47:712–721. https://doi.org/10.1093/schbul/sbaa157
North HF, Bruggemann J, Cropley V et al (2021) Increased peripheral inflammation in schizophrenia is associated with worse cognitive performance and related cortical thickness reductions. Eur Arch Psychiatry Clin Neurosci 271:595–607. https://doi.org/10.1007/S00406-021-01237-Z
de Bie J, Lim CK, Guillemin GJ (2016) Progesterone alters kynurenine pathway activation in IFN-γ-activated macrophages—relevance for neuroinflammatory diseases. Int J Tryptophan Res 9:89–93. https://doi.org/10.4137/IJTR.S40332
Acknowledgements
Dr. Stefano Comai and Dr. Marta Bosia are funded by the Italian Ministry of Health (Grant No. GR-2019-12369523) and Dr. Stefano Comai is also funded by the Department of Pharmaceutical and Pharmacological Sciences, University of Padova (Grant No. PRID-J 2021). Prof. Gilles J. Guillemin is funded by the National Health and Medical Research Council (NHMRC) and Macquarie University.
Funding
The authors did not receive support from any organization for the submitted work. No funding was received to assist with the preparation of this manuscript. No funding was received for conducting this study. No funds, grants, or other support was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflict of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sapienza, J., Spangaro, M., Guillemin, G.J. et al. Importance of the dysregulation of the kynurenine pathway on cognition in schizophrenia: a systematic review of clinical studies. Eur Arch Psychiatry Clin Neurosci 273, 1317–1328 (2023). https://doi.org/10.1007/s00406-022-01519-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01519-0