Abstract
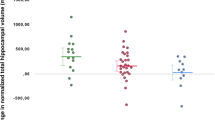
Vascular endothelial growth factor (VEGF) is involved in the development of major depressive disorder (MDD). Recently, a genome-wide association study has revealed that four VEGF-related single nucleotide polymorphisms (SNPs) (i.e., rs4416670, rs6921438, rs6993770 and rs10738760) were independently associated with circulating VEGF levels. The current study investigated the relationship between brain volume and these four SNPs in first-episode drug-naïve MDD patients. A total of 38 first-episode drug-naïve MDD patients and 39 healthy subjects (HS) were recruited and underwent high-resolution T1-weighted imaging. Blood samples were collected from all the participants for serum VEGF assays and VEGF-related SNPs genotyping. Genotype–diagnosis interactions related to whole-brain cortical thickness and hippocampal subfield volumes were evaluated for the four SNPs. The results revealed a genotype–diagnosis interaction only for rs6921438 (i.e., the MDD patients and HS with the G/G genotype versus the MDD patients and HS with A-carrier genotype) in the subiculum of the left hippocampus (p < 0.05), and not the other SNPs. There was a volume reduction in the left subiculum of G/G genotype patients compared with the other groups. The “hypochondriasis” scores of the HAMD-17 scale were significantly higher in the G/G genotype patients than the A-carrier genotype patients. The association was observed between VEGF-related SNP rs6921438 and subiculum atrophy in first-episode drug-naïve MDD patients.

Similar content being viewed by others
References
Folkman J, D’Amore PA (1996) Blood vessel formation: what is its molecular basis? Cell 87(7):1153–1155
Storkebaum E, Lambrechts D, Carmeliet P (2004) VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 26(9):943–954. https://doi.org/10.1002/bies.20092
Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA (2002) Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA 99(18):11946–11950. https://doi.org/10.1073/pnas.182296499
Sondell M, Lundborg G, Kanje M (1999) Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 19(14):5731–5740
Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E (2011) Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci USA 108(12):5081–5086. https://doi.org/10.1073/pnas.1007640108
McCloskey DP, Croll SD, Scharfman HE (2005) Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci 25(39):8889–8897. https://doi.org/10.1523/JNEUROSCI.2577-05.2005
Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M (2001) Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J 15(7):1218–1220
Clark-Raymond A, Halaris A (2013) VEGF and depression: a comprehensive assessment of clinical data. J Psychiatr Res 47(8):1080–1087. https://doi.org/10.1016/j.jpsychires.2013.04.008
Sharma AN, da Costa e Silva BF, Soares JC, Carvalho AF, Quevedo J (2016) Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J Affect Disord 197:9–20. https://doi.org/10.1016/j.jad.2016.02.067
Iga J, Ueno S, Yamauchi K, Numata S, Tayoshi-Shibuya S, Kinouchi S, Nakataki M, Song H, Hokoishi K, Tanabe H, Sano A, Ohmori T (2007) Gene expression and association analysis of vascular endothelial growth factor in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 31(3):658–663. https://doi.org/10.1016/j.pnpbp.2006.12.011
Lee BH, Kim YK (2012) Increased plasma VEGF levels in major depressive or manic episodes in patients with mood disorders. J Affect Disord 136(1–2):181–184. https://doi.org/10.1016/j.jad.2011.07.021
Duric V, Duman RS (2013) Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci 70(1):39–53. https://doi.org/10.1007/s00018-012-1020-7
Debette S, Visvikis-Siest S, Chen MH, Ndiaye NC, Song C, Destefano A, Safa R, Azimi Nezhad M, Sawyer D, Marteau JB, Xanthakis V, Siest G, Sullivan L, Pfister M, Smith H, Choi SH, Lamont J, Lind L, Yang Q, Fitzgerald P, Ingelsson E, Vasan RS, Seshadri S (2011) Identification of cis- and trans-acting genetic variants explaining up to half the variation in circulating vascular endothelial growth factor levels. Circ Res 109(5):554–563. https://doi.org/10.1161/CIRCRESAHA.111.243790
Xie T, Stathopoulou MG, de Andres F, Siest G, Murray H, Martin M, Cobaleda J, Delgado A, Lamont J, Penas LE, Visvikis-Siest ALL S (2017) VEGF-related polymorphisms identified by GWAS and risk for major depression. Transl Psychiatry 7(3):e1055. https://doi.org/10.1038/tp.2017.36
Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, Loehrer E, Tiemeier H, Hofman A, Niessen WJ, Vernooij MW, Ikram MA, Wittfeld K, Grabe HJ, Block A, Hegenscheid K, Volzke H, Hoehn D, Czisch M, Lagopoulos J, Hatton SN, Hickie IB, Goya-Maldonado R, Kramer B, Gruber O, Couvy-Duchesne B, Renteria ME, Strike LT, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Wright MJ, Hall GB, MacQueen GM, Frey EM, Carballedo A, van Velzen LS, van Tol MJ, van der Wee NJ, Veer IM, Walter H, Schnell K, Schramm E, Normann C, Schoepf D, Konrad C, Zurowski B, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Sussmann JE, Godlewska BR, Cowen PJ, Fischer FH, Rose M, Penninx BW, Thompson PM, Hibar DP (2016) Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 21(6):806–812. https://doi.org/10.1038/mp.2015.69
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TGM, Bos D, Ikram MA, Vernooij MW, Niessen WJ, Tiemeier H, Hofman A, Wittfeld K, Grabe HJ, Janowitz D, Bulow R, Selonke M, Volzke H, Grotegerd D, Dannlowski U, Arolt V, Opel N, Heindel W, Kugel H, Hoehn D, Czisch M, Couvy-Duchesne B, Renteria ME, Strike LT, Wright MJ, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Goya-Maldonado R, Gruber O, Kramer B, Hatton SN, Lagopoulos J, Hickie IB, Frodl T, Carballedo A, Frey EM, van Velzen LS, Penninx B, van Tol MJ, van der Wee NJ, Davey CG, Harrison BJ, Mwangi B, Cao B, Soares JC, Veer IM, Walter H, Schoepf D, Zurowski B, Konrad C, Schramm E, Normann C, Schnell K, Sacchet MD, Gotlib IH, MacQueen GM, Godlewska BR, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Hall J, Sussmann JE, Li M, Walter M, Aftanas L, Brack I, Bokhan NA, Thompson PM, Veltman DJ (2017) Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 22(6):900–909. https://doi.org/10.1038/mp.2016.60
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65(1):7–19. https://doi.org/10.1016/j.neuron.2009.11.031
Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, Kumar A (2008) Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am J Psychiatry 165(2):229–237. https://doi.org/10.1176/appi.ajp.2007.07030506
Cao B, Passos IC, Mwangi B, Amaral-Silva H, Tannous J, Wu MJ, Zunta-Soares GB, Soares JC (2017) Hippocampal subfield volumes in mood disorders. Mol Psychiatry 22(9):1352–1358. https://doi.org/10.1038/mp.2016.262
Cole J, Toga AW, Hojatkashani C, Thompson P, Costafreda SG, Cleare AJ, Williams SC, Bullmore ET, Scott JL, Mitterschiffthaler MT, Walsh ND, Donaldson C, Mirza M, Marquand A, Nosarti C, McGuffin P, Fu CH (2010) Subregional hippocampal deformations in major depressive disorder. J Affect Disord 126(1–2):272–277. https://doi.org/10.1016/j.jad.2010.03.004
Frey BN, Andreazza AC, Nery FG, Martins MR, Quevedo J, Soares JC, Kapczinski F (2007) The role of hippocampus in the pathophysiology of bipolar disorder. Behav Pharmacol 18(5–6):419–430. https://doi.org/10.1097/FBP.0b013e3282df3cde
Posener JA, Wang L, Price JL, Gado MH, Province MA, Miller MI, Babb CM, Csernansky JG (2003) High-dimensional mapping of the hippocampus in depression. Am J Psychiatry 160(1):83–89. https://doi.org/10.1176/appi.ajp.160.1.83
Tae WS, Kim SS, Lee KU, Nam EC, Choi JW, Park JI (2011) Hippocampal shape deformation in female patients with unremitting major depressive disorder. AJNR Am J Neuroradiol 32(4):671–676. https://doi.org/10.3174/ajnr.A2367
Campbell S, Marriott M, Nahmias C, MacQueen GM (2004) Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 161(4):598–607. https://doi.org/10.1176/appi.ajp.161.4.598
Molendijk ML, van Tol MJ, Penninx BW, van der Wee NJ, Aleman A, Veltman DJ, Spinhoven P, Elzinga BM (2012) BDNF val66met affects hippocampal volume and emotion-related hippocampal memory activity. Transl Psychiatry 2:e74. https://doi.org/10.1038/tp.2011.72
Ide S, Kakeda S, Watanabe K, Yoshimura R, Abe O, Hayashi K, Ueda I, Kishi T, Katsuki A, Umene-Nakano W, Iwata N, Nakamura J, Korogi Y (2015) Relationship between a BDNF gene polymorphism and the brain volume in treatment-naive patients with major depressive disorder: A VBM analysis of brain MRI. Psychiatry Res 233(2):120–124. https://doi.org/10.1016/j.pscychresns.2015.05.016
Igata N, Kakeda S, Watanabe K, Ide S, Kishi T, Abe O, Igata R, Katsuki A, Iwata N, Yoshimura R, Korogi Y (2017) Voxel-based morphometric brain comparison between healthy subjects and major depressive disorder patients in Japanese with the s/s genotype of 5-HTTLPR. Sci Rep 7(1):3931. https://doi.org/10.1038/s41598-017-04347-8
Pan CC, McQuoid DR, Taylor WD, Payne ME, Ashley-Koch A, Steffens DC (2009) Association analysis of the COMT/MTHFR genes and geriatric depression: an MRI study of the putamen. Int J Geriatr Psychiatry 24(8):847–855. https://doi.org/10.1002/gps.2206
Watanabe K, Kakeda S, Yoshimura R, Abe O, Ide S, Hayashi K, Katsuki A, Umene-Nakano W, Watanabe R, Nakamura J, Korogi Y (2015) Relationship between the catechol-O-methyl transferase Val108/158Met genotype and brain volume in treatment-naive major depressive disorder: Voxel-based morphometry analysis. Psychiatry Res 233(3):481–487. https://doi.org/10.1016/j.pscychresns.2015.07.024
Ueda I, Kakeda S, Watanabe K, Yoshimura R, Kishi T, Abe O, Ide S, Moriya J, Katsuki A, Hori H, Iwata N, Nakamura J, Korogi Y (2016) Relationship between G1287A of the NET Gene Polymorphisms and Brain Volume in Major Depressive Disorder: A Voxel-Based MRI Study. PLoS One 11(3):e0150712. https://doi.org/10.1371/journal.pone.0150712
Igata R, Katsuki A, Kakeda S, Watanabe K, Igata N, Hori H, Konishi Y, Atake K, Kawasaki Y, Korogi Y, Yoshimura R (2017) PCLO rs2522833-mediated gray matter volume reduction in patients with drug-naive, first-episode major depressive disorder. Transl Psychiatry 7(5):e1140. https://doi.org/10.1038/tp.2017.100
Hamilton M (1967) Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6(4):278–296
Serretti A, Cusin C, Lattuada E, Bella DD, Catalano M, Smeraldi E (1999) Serotonin transporter gene (5-HTTLPR) is not associated with depressive symptomatology in mood disorders. Molecular Psychiatry 4:280. https://doi.org/10.1038/sj.mp.4000485
Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, Fischl B, Dale A (2006) Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30(2):436–443. https://doi.org/10.1016/j.neuroimage.2005.09.046
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17(1):87–97. https://doi.org/10.1109/42.668698
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9(2):179–194. https://doi.org/10.1006/nimg.1998.0395
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B (2009) Automated segmentation of hippocampal subfields from ultra-high resolution in vivo MRI. Hippocampus 19(6):549–557. https://doi.org/10.1002/hipo.20615
Iglesias JE, Augustinack JC, Nguyen K, Player CM, Player A, Wright M, Roy N, Frosch MP, McKee AC, Wald LL, Fischl B, Van Leemput K, Alzheimer’s Disease Neuroimaging I (2015) A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 115:117–137. https://doi.org/10.1016/j.neuroimage.2015.04.042
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S, Williams SC (2011) Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68(7):675–690. https://doi.org/10.1001/archgenpsychiatry.2011.60
Chen MC, Hamilton JP, Gotlib IH (2010) Decreased hippocampal volume in healthy girls at risk of depression. Arch Gen Psychiatry 67(3):270–276. https://doi.org/10.1001/archgenpsychiatry.2009.202
Brown ES, Hughes CW, McColl R, Peshock R, King KS, Rush AJ (2014) Association of depressive symptoms with hippocampal volume in 1936 adults. Neuropsychopharmacology 39(3):770–779. https://doi.org/10.1038/npp.2013.271
Moser MB, Moser EI (1998) Functional differentiation in the hippocampus. Hippocampus 8(6):608–619
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G (2004) Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 56(9):640–650. https://doi.org/10.1016/j.biopsych.2004.08.022
Rosoklija G, Toomayan G, Ellis SP, Keilp J, Mann JJ, Latov N, Hays AP, Dwork AJ (2000) Structural abnormalities of subicular dendrites in subjects with schizophrenia and mood disorders: preliminary findings. Arch Gen Psychiatry 57(4):349–356
Willard SL, Riddle DR, Forbes ME, Shively CA (2013) Cell number and neuropil alterations in subregions of the anterior hippocampus in a female monkey model of depression. Biol Psychiatry 74(12):890–897. https://doi.org/10.1016/j.biopsych.2013.03.013
Isikli S, Ugurlu O, Durmusoglu E, Kizilates G, Kitis O, Ozan E, Eker C, Coburn K, Gonul AS (2013) Altered hippocampal formation shape in first-episode depressed patients at 5-year follow-up. J Psychiatr Res 47(1):50–55. https://doi.org/10.1016/j.jpsychires.2012.08.022
Watanabe R, Kakeda S, Watanabe K, Liu X, Katsuki A, Umeno-Nakano W, Hori H, Abe O, Yoshimura R, Korogi Y (2017) Relationship between the hippocampal shape abnormality and serum cortisol levels in first-episode and drug-naive major depressive disorder patients. Depress Anxiety 34(5):401–409. https://doi.org/10.1002/da.22604
Choi SH, Ruggiero D, Sorice R, Song C, Nutile T, Vernon Smith A, Concas MP, Traglia M, Barbieri C, Ndiaye NC, Stathopoulou MG, Lagou V, Maestrale GB, Sala C, Debette S, Kovacs P, Lind L, Lamont J, Fitzgerald P, Tonjes A, Gudnason V, Toniolo D, Pirastu M, Bellenguez C, Vasan RS, Ingelsson E, Leutenegger AL, Johnson AD, DeStefano AL, Visvikis-Siest S, Seshadri S, Ciullo M (2016) Six novel loci associated with circulating VEGF levels identified by a meta-analysis of genome-wide association studies. PLoS Genet 12(2):e1005874. https://doi.org/10.1371/journal.pgen.1005874
Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ (2004) VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36(8):827–835. https://doi.org/10.1038/ng1395
de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT (1992) The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255(5047):989–991
Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT (1993) Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA 90(16):7533–7537
Yang SZ, Zhang LM, Huang YL, Sun FY (2003) Distribution of Flk-1 and Flt-1 receptors in neonatal and adult rat brains. Anat Rec A Discov Mol Cell Evol Biol 274(1):851–856. https://doi.org/10.1002/ar.a.10103
Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ (2005) Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 21(5):1304–1314. https://doi.org/10.1111/j.1460-9568.2005.03951.x
Adhikari A, Topiwala MA, Gordon JA (2010) Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65(2):257–269. https://doi.org/10.1016/j.neuron.2009.12.002
Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ (2012) Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76(4):804–812. https://doi.org/10.1016/j.neuron.2012.09.028
Kahl KG, Bens S, Ziegler K, Rudolf S, Kordon A, Dibbelt L, Schweiger U (2009) Angiogenic factors in patients with current major depressive disorder comorbid with borderline personality disorder. Psychoneuroendocrinology 34(3):353–357. https://doi.org/10.1016/j.psyneuen.2008.09.016
Acknowledgements
This study was supported by a Health and Labor Research Grant in Japan (#1401010101).
Funding
This study did not receive any specific grant from any funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have reported no potential conflicts of interest. The authors have completed the form for the disclosure of potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Nguyen, L., Kakeda, S., Katsuki, A. et al. Relationship between VEGF-related gene polymorphisms and brain morphology in treatment-naïve patients with first-episode major depressive disorder. Eur Arch Psychiatry Clin Neurosci 269, 785–794 (2019). https://doi.org/10.1007/s00406-018-0953-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-018-0953-8