Abstract
Purpose
The purpose of this review is to systematically summarize the application of organoids in the field of otolaryngology and head and neck surgery. It aims to shed light on the current advancements and future potential of organoid technology in these areas, particularly in addressing challenges like hearing loss, cancer research, and organ regeneration.
Methods
Review of current literature regrading organoids in the field of otolaryngology and head and neck surgery.
Results
The review highlights several advancements in the field. In otology, the development of organoid replacement therapies offers new avenues for treating hearing loss. In nasal science, the creation of specific organoid models aids in studying nasopharyngeal carcinoma and respiratory viruses. In head and neck surgery, innovative approaches for squamous cell carcinoma prediction and thyroid regeneration using organoids have been developed.
Conclusion
Organoid research in otolaryngology—head and neck surgery is still at an early stage. This review underscores the potential of this technology in advancing our understanding and treatment of various conditions, predicting a transformative impact on future medical practices in these fields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Definition and characteristics of organoids
Organoids, as miniature tissue and organ analogs, have three characteristics, including self-assembly, various cell types, and similar to the internal organs in structure and function to a great extent [1]. Organoids are cultured in vitro with 3D technology, in which multicellular masses can highly simulate the physiological and pathological structure and tumor cell heterogeneity of tissues or organs in vivo [2, 3].
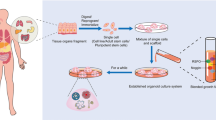
As organoids of various organs and tissues, such as intestines, stomach, liver and kidney [4,5,6,7], have been successfully cultured in vitro, the huge potential of organoid technology has been continuously developed. Organoid technology provides in vitro conditions for understanding the mechanism of development of tissues and organs, disease development and precision medicine. In addition, it can be used for drug toxicity detection, efficacy evaluation and new drug screening. However, there is still a lack of systematic synthesis of studies on organoid technology in otolaryngology, head and neck surgery. Therefore, this paper summarized the latest research results of organoids in otolaryngology—head and neck surgery and discussed the future development of organoid technology. The acquisition, construction and application of organoids are shown in Fig. 1.
Establishment of organoid model
Organoids are mainly cultured from stem cells, including pluripotent stem cells, adult stem cells and tumor stem cells. Presently, most organoid modeling methods require stem cells, matrigel and cytokine-rich medium, which can be established in an average of 10–14 days. The organoid construction process is shown in Fig. 2.
Preparation of tissue
Organoids can be produced from solid materials, such as surgical specimens, puncture biopsy specimens and nasal brush specimens, or liquids like urine, ascites and bronchoalveolar lavage fluid [8,9,10,11]. For solid specimens, the first step is to remove the non-epithelial tissue (such as muscle or fat) as much as possible, then use a scalpel to cut it into 1–3-mm small pieces, digest the tissue with enzymes and separate the epithelial cells.
Inoculation of cells
The isolated cells or small cell masses are inoculated into 3D extracellular matrix (ECM) hydrogels, such as basement membrane extract (BME), Matrigel or Geltrex, which can be used as artificial lamina propria [12].
Organoid culture
After inoculation, cells are supplemented with a medium consisting of a mixture of growth factors that trigger regenerative or damage responses in epithelial tissue stem cells. The key components include: (i) activators of Wnt signaling, such as Wnt ligand and LGR5 ligand R-spondin (RSPO) [13,14,15]; (ii) tyrosine receptor kinase ligands, such as epidermal growth factor (EGF), capable of promoting epithelial cell value addition [16, 17]; and (iii) inhibitors of the transforming growth factor-β/osteogenic protein signaling pathway, such as Noggin [18], which induces epithelial differentiation.
Current research
Otology research
More than 6% of the world's population suffers from hearing loss and balance impairment [19]. Both sensory systems are located in the inner ear and can be affected by aging, genetic mutations, infections, noise exposure and ototoxic drugs. Hearing loss is irreversible, and there are currently no medications that specifically target sensory recovery. As a 3D multicellular system that simulates the inner ear in vitro, inner ear organoids are promising new tools to realize cell replacement therapy and understand the inner ear nerve cells [20, 21].
Culture of inner ear organoids from pluripotent stem cells
Unlike other organoids, the inner ear is difficult to biopsy and grow for a long time [22], so patient-sourced tissue cannot be used, and using fetal-sourced tissue has ethical issues. Therefore, human pluripotent stem cells (hPSCs) may be a potential source of tissue cells for experiments.
hPSCs differentiate into ear progenitor cells and more mature inner ear cells by mimicking embryonic and fetal development [11, 23, 24]. In embryos, the development of the inner ear requires the participation of multiple cell types from multiple cell lineages, including inner ear epithelial cells, neuron cells and glial cells from the ectoderm, and periauricular mesenchymal cells from the mesoderm [25]. The challenge is synthesizing these multicell lines into an inner ear organoid in vitro, which is a long-term bioengineering challenge.
As an extremely complex organ, the inner ear is formed by integrating many signal pathways across space and time. These signals come from the inner and surrounding tissues of the epithelial cells, which make the cochlear progenitor cells differentiate into cochlear and vestibular cells. Most of our knowledge of these mechanisms comes from animal models, and very little has been done on human fetal inner ear tissue [26]. To some extent, the self-assembly of inner ear epithelial cells and neuronal complexes can be stimulated by using recombinant proteins and small molecules to simulate signals in hPSC 3D culture [20]. However, this approach is difficult to control, and the resulting organoids are of irregular shape and size and contain an unpredictable mix of sensory and non-sensory cells. In future studies, more sophisticated 3D bioprinting-based or microfluid-based approaches may be needed to build spatially controlled cell structures that can be affected by signal gradients to create an inner ear organoid chip.
Recent studies have found that the use of microfluidics or microwell systems to enable hPSCs to form embryonic-like, renal or intestinal structures can guide studies to induce inner ear formation [9, 27,28,29].
Inner ear organoids simulate hereditary deafness
It is estimated that 430 million people worldwide suffer from moderate to severe hearing loss [30, 31]. The most permanent hearing loss is of the sensorineural type (SNHL), and the causes include aging [32], infection [33, 34], noise [35, 36], ototoxic drug [37, 38], traumatic tympanic membrane rupture [39] and single gene mutation.
Although the etiology of SNHL is largely established, its underlying pathophysiological mechanisms have not been fully elucidated at the cellular and molecular levels. Therefore, the use of inner ear organoids to model hereditary deafness is a very valuable application.
There are generally two approaches to in vitro modeling of hereditary deafness. The first involves using CRISPR-Cas9 to introduce deafness-related mutations into wild embryonic stem cell (ESC) lines [40, 41], guided editing [42] or other precision genome editing techniques. The second is to obtain somatic cells from patients with inherited deafness and induce them to be transformed into induced pluripotent stem cells (iPSCs) [43, 44] and then gradually induce iPSCs or CrisPR-Cas9-edited ESCs to differentiate into inner ear-like tissues. The use of iPSCs clearly has greater therapeutic potential than ESCs, as the use of IPSC-derived donor cells in the treatment of the inner ear can avoid rejection.
Researchers have modeled two types of autosomal recessive non-syndromic deafness, DFNB2 and DFNB3, using a 2D culture system based on hiPSC [45, 46]. However, the organoid-based 3D culture system can easily perform single-cell RNA sequencing (RNA-SEQ) on inner ear-like tissues. Tang et al. investigated human hearing loss caused by mutations in the gene encoding type II transmembrane protease 3 (TMPRSS3) using inner ear organoid and scRNA-seq, revealing a potential role for calcium homeostasis and extracellular matrix maintenance in TMPRSS3-associated deafness [47].
Rhinology research
Nasopharyngeal carcinoma organoid disease model
The nasopharyngeal carcinoma (NPC) is a tailor-made malignant tumor with a high geographical prevalence in the top and lateral walls of the nasopharynx and is closely correlated with Epstein–Barr virus infection. For patients with NPC at the beginning of stage I, II, III and IV, the 5-year survival rate is more than 70% after comprehensive treatment with radiotherapy and chemotherapy [48], but 25% of NPC patients fail due to local recurrence and distant metastasis [49]. Studies have shown that tumor stem cells are closely related to the occurrence, development, recurrence and metastasis of cancer. They can not only self-renew but also have the resistance effect to traditional chemoradiotherapy. Therefore, it is valuable to screen out chemotherapeutic drugs that NPC stem cells sensitive to.
Tumor organoids are the tumor tissues of patients cultured in vitro in 3D, and tumor cells with the potential of stem cells will converge and grow spherical, thus forming organoids with the ability of self-renewal and self-organization. Compared with traditional cell lines and xenotransplantation, tumor organoids have the advantage of individualization. In addition, the tissue needs are less, and the culture cycle is shorter. It is helpful to screen chemotherapy drugs or targeted drugs accurately and efficiently [50]. However, it does not have a vascular and immune environment, so it still has limitation when used to screen immunotherapy drugs.
Patient-derived xenografts (PDXs) have been used in NPC studies, but the low success rate and high cost of PDXs limit their large-scale application [51, 52]. NPC tissues are usually obtained by endoscopic biopsy, and their small tissue size and poor cell activity pose major challenges in Nasopharyngeal carcinoma organoid (NPCO) culture. Wang et al. established a patient-derived organoid model, and an optimized medium can significantly increase the success rate of NPCO culture, preserve parental tumor heterogeneity and reproduce its pathophysiological features [53].
However, there are still many challenges to generating NPCOs, including the overgrowth of fibroblasts. There are many infiltrating lymphocytes in NPC tissues [54]. It has been reported that T and B lymphocytes can secrete cytokines to control the growth of fibroblasts [55, 56]. However, after several passages, the immune cells in NPCOs would stop growing and die, resulting in decreased concentrations of TGF-β and TNF-α, which could not inhibit fibroblast growth [57]. Further studies are needed to understand how to inhibit the growth of fibroblasts to prolong the passage of NPCOs.
Nasal organoid respiratory virus model
Respiratory organoids are often used as an in vitro airway model to study the pathogenesis of respiratory viruses and test therapeutic methods [58]. However, respiratory organoids technology needs to use invasive methods to obtain patient samples. Rajan et al. reported a non-invasive technique using human nasal organoids (HNOs) as an alternative to tissue-derived organoids [59].
HNOs were cultured in an air–liquid interface (ALI), and the infection of two major human respiratory viruses, including respiratory syncytial virus (RSV) and novel coronavirus (SARS-CoV-2), was evaluated, reproducing the complex host-virus interaction. SARS-CoV-2 causes severe damage to cilia and epithelial cells [60,61,62], no interferon-I response and little mucus secretion. In contrast, RSV causes mucous hypersecretion and severe interferon-I response with ciliary body damage.
Chiu et al. also reported the establishment of a nasal organoid model to study the infection of SARS-CoV-2. They further reproduced the higher infectability and replication adaptability of the Omicron variant showing its pathogenesis, such as the destruction of ciliary cells and tight connections, to promote the spread and development of the virus [63].
The nasal organoid respiratory virus model, which simulates upper respiratory tract infections and effectively reconstructs human nasal epithelium in a stable culture plate, provides microbiologists with a powerful and convenient tool to study the pathogenesis and test treatments for the current epidemic of SARS-CoV-2 and its emerging variants.
Pharyngology, head and neck surgery research
Head and neck squamous cell carcinoma prediction model
Head and neck malignancies are the seventh most common tumor types worldwide, among which more than 90% are [64]. The emergence of therapies, including targeted therapies and immunotherapies, is increasing the need to test treatment options in personalized settings. Currently, new treatments for HNSCC are mainly tested at the population level, meaning that within a group of patients, multiple subgroups with different efficacy and side effects are included. This makes it difficult to predict how well a therapy will work for an individual patient. Consequently, new therapies are often only tested as palliative treatments in patients with advanced HNSCC.
HNSCC organoids fill the gap in personalized prediction of treatment outcomes. In a specific culture environment, tumor tissue can be grown into organoid models to test different treatments, predict treatment outcomes, and then treat patients. In addition, it could allow new drugs to be tested before entering clinical practice.
Tissue-derived thyroid organoid model
Total thyroidectomy as a treatment for thyroid cancer can cause hypothyroidism and require patients to take thyroid hormones for life. Ogundipe et al. isolated cells from mouse and human thyroid tissue and developed an in vitro 3D culture system [65]. It was demonstrated that both mouse and human thyroid cells could be isolated, expanded in vitro and cultured for a long time. Furthermore, these cells were able to self-renew and differentiate in vitro, suggesting that these cells had the proliferative capacity required for expansion. After transplanting a few cells, these organoids formed fully functioning hormone-producing thyroid follicles in mice with hypothyroidism.
There are two important aspects of thyroid organoid culture that warrant further investigation with this technique. Firstly, organoids can be amplified while maintaining genetic stability [66], proving that they have certain safety as grafts. Secondly, thyroid organoids of patients who have undergone radical surgery for thyroid cancer can be generated from cryo-stored stem cells, which can come from bone marrow or adipose tissue [67].
Conclusions
The establishment of various organoid models provides new models in vitro for drug research, disease research and organ replacement therapy, with great potential. However, its research in otolaryngology—head and neck surgery is still at an early stage, and its application is relatively limited. Future studies should consider expanding the application scope of this technology, including providing personalized drug treatment for various tumors through tumor organoid technology. Furthermore, by combining organoid technology with 3D printing technology, organoid technology is more closely associated with the regeneration of various organ tissues. It is believed that further development of otolaryngology—head and neck organoid culture systems will have a huge prospect in the future of basic research and translational medicine.
Availability of data and materials
Not applicable.
Abbreviations
- ECM:
-
Extracellular matrix
- BME:
-
Basement membrane extract
- RSPO:
-
R-spondin
- EGF:
-
Epidermal growth factor
- hPSCs:
-
Human pluripotent stem cells
- SNHL:
-
Sensorineural type hearing loss
- ESC:
-
Embryonic stem cell
- iPSCs:
-
Induced pluripotent stem cells
- RNA-SEQ:
-
RNA sequencing
- TMPRSS3:
-
Transmembrane protease 3
- NPC:
-
Nasopharyngeal carcinoma
- PDXs:
-
Patient-derived xenografts
- NPCO:
-
Nasopharyngeal carcinoma organoid
- HNOs:
-
Human nasal organoids
- ALI:
-
Air–liquid interface
- RSV:
-
Respiratory syncytial virus
- SARS-CoV-2:
-
Novel coronavirus
- HNSCC:
-
Head and neck squamous cell carcinoma
References
Kretzschmar K, Clevers H (2016) Organoids: modeling development and the stem cell niche in a dish. Dev Cell 38(6):590–600
Drost J, Clevers H (2018) Organoids in cancer research. Nat Rev Cancer 18(7):407–418
Rossi G, Manfrin A, Lutolf MP (2018) Progress and potential in organoid research. Nat Rev Genet 19(11):671–687
Ray K (2020) Next-generation intestinal organoids. Nat Rev Gastroenterol Hepatol 17(11):649
Ray K (2017) Development: modelling human stomach development with gastric organoids. Nat Rev Gastroenterol Hepatol 14(2):68
Hu H et al (2018) Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175(6):1591-1606 e19
Nishinakamura R (2019) Human kidney organoids: progress and remaining challenges. Nat Rev Nephrol 15(10):613–624
Geurts MH et al (2020) CRISPR-based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. Cell Stem Cell 26(4):503-510 e7
Schutgens F et al (2019) Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol 37(3):303–313
Kopper O et al (2019) An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med 25(5):838–849
Sachs N et al (2019) Long-term expanding human airway organoids for disease modeling. EMBO J 38(4):e100300
Lee GY et al (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4(4):359–365
Sato T et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459(7244):262–265
Ootani A et al (2009) Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15(6):701–706
de Lau W et al (2011) Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476(7360):293–297
Rheinwald JG, Green H (1977) Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 265(5593):421–424
Cancer Genome Atlas N. (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517(7536):576–582
Haramis AP et al (2004) De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science 303(5664):1684–1686
Hulse R et al (2019) Peripheral vestibular disorders: an epidemiologic survey in 70 million individuals. Otol Neurotol 40(1):88–95
Koehler KR et al (2017) Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol 35(6):583–589
Roccio M, Edge ASB (2019) Inner ear organoids: new tools to understand neurosensory cell development, degeneration and regeneration. Development 146(17):dev177188
Taylor RR et al (2015) Characterizing human vestibular sensory epithelia for experimental studies: new hair bundles on old tissue and implications for therapeutic interventions in ageing. Neurobiol Aging 36(6):2068–2084
Czajkowski A et al (2019) Pluripotent stem cell-derived cochlear cells: a challenge in constant progress. Cell Mol Life Sci 76(4):627–635
Tang PC, Hashino E, Nelson RF (2020) Progress in modeling and targeting inner ear disorders with pluripotent stem cells. Stem Cell Rep 14(6):996–1008
van der Valk WH et al (2021) Building inner ears: recent advances and future challenges for in vitro organoid systems. Cell Death Differ 28(1):24–34
Roccio M et al (2018) Molecular characterization and prospective isolation of human fetal cochlear hair cell progenitors. Nat Commun 9(1):4027
Zheng Y et al (2019) Controlled modelling of human epiblast and amnion development using stem cells. Nature 573(7774):421–425
Manfrin A et al (2019) Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat Methods 16(7):640–648
Brandenberg N et al (2020) High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng 4(9):863–874
Collaborators GBDHL (2021) Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet 397(10278):996–1009
Goman AM, Lin FR (2016) Prevalence of hearing loss by severity in the United States. Am J Public Health 106(10):1820–1822
Elliott KL et al (2022) Age-related hearing loss: sensory and neural etiology and their interdependence. Front Aging Neurosci 14:814528
Goderis J et al (2014) Hearing loss and congenital CMV infection: a systematic review. Pediatrics 134(5):972–982
Brown ED et al (2009) A systematic review of neonatal toxoplasmosis exposure and sensorineural hearing loss. Int J Pediatr Otorhinolaryngol 73(5):707–711
Lie A et al (2016) Occupational noise exposure and hearing: a systematic review. Int Arch Occup Environ Health 89(3):351–372
Hormozi M et al (2017) The risk of hearing loss associated with occupational exposure to organic solvents mixture with and without concurrent noise exposure: a systematic review and meta-analysis. Int J Occup Med Environ Health 30(4):521–535
Farzal Z et al (2016) The role of routine hearing screening in children with cystic fibrosis on aminoglycosides: a systematic review. Laryngoscope 126(1):228–235
Frisina RD et al (2016) Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol 34(23):2712–2720
Honeybrook A et al (2017) Hearing and mortality outcomes following temporal bone fractures. Craniomaxillofac Trauma Reconstr 10(4):281–285
Cong L et al (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339(6121):819–823
Mali P et al (2013) RNA-guided human genome engineering via Cas9. Science 339(6121):823–826
Anzalone AV et al (2019) Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576(7785):149–157
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Takahashi K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Tang ZH et al (2016) Genetic correction of induced pluripotent stem cells from a deaf patient with MYO7A mutation results in morphologic and functional recovery of the derived hair cell-like cells. Stem Cells Transl Med 5(5):561–571
Chen JR et al (2016) Effects of genetic correction on the differentiation of hair cell-like cells from iPSCs with MYO15A mutation. Cell Death Differ 23(8):1347–1357
Tang PC et al (2019) Defective Tmprss3-associated hair cell degeneration in inner ear organoids. Stem Cell Rep 13(1):147–162
Sun X et al (2014) Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 110(3):398–403
Chung AK et al (2019) Targeted sequencing of cancer-related genes in nasopharyngeal carcinoma identifies mutations in the TGF-beta pathway. Cancer Med 8(11):5116–5127
Vlachogiannis G et al (2018) Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359(6378):920–926
Vaes RDW et al (2020) Generation and initial characterization of novel tumour organoid models to study human pancreatic cancer-induced cachexia. J Cachexia Sarcopenia Muscle 11(6):1509–1524
Costales-Carrera A et al (2019) Plocabulin displays strong cytotoxic activity in a personalized colon cancer patient-derived 3D organoid assay. Mar Drugs 17(11):648
Wang XW et al (2022) Establishment of a patient-derived organoid model and living biobank for nasopharyngeal carcinoma. Ann Transl Med 10(9):526
Ding RB et al (2021) Molecular landscape and subtype-specific therapeutic response of nasopharyngeal carcinoma revealed by integrative pharmacogenomics. Nat Commun 12(1):3046
Oka T, Komuro I (2012) Molecular and cellular mechanisms of organ fibrosis. Nihon Rinsho 70(9):1510–1516
Yu Q et al (2005) A role for T lymphocytes in mediating cardiac diastolic function. Am J Physiol Heart Circ Physiol 289(2):H643–H651
Schaffer M, Barbul A (1998) Lymphocyte function in wound healing and following injury. Br J Surg 85(4):444–460
Zhou J et al (2018) Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci U S A 115(26):6822–6827
Rajan A et al (2021) The human nose organoid respiratory virus model: an ex-vivo human challenge model to study RSV and SARS-CoV-2 pathogenesis and evaluate therapeutics. bioRxiv
Ahn JH et al (2021) Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19. J Clin Invest 131(13):e148517
Robinot R et al (2021) SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance. Nat Commun 12(1):4354
Zhu N et al (2020) Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells. Nat Commun 11(1):3910
Chiu MC et al (2022) Human nasal organoids model SARS-CoV-2 upper respiratory infection and recapitulate the differential infectivity of emerging variants. MBio 13(4):e0194422
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Ogundipe VML et al (2021) Generation and differentiation of adult tissue-derived human thyroid organoids. Stem Cell Rep 16(4):913–925
Sato T et al (2011) Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141(5):1762–1772
Harris DT (2014) Stem cell banking for regenerative and personalized medicine. Biomedicines 2(1):50–79
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Zhejiang Provincial (grant nos. LY20H130001, LQ21H130001), Ningbo Health Branding Subject Fund (grant no. PPXK2018-02), Ningbo Natural Science Foundation (grant no. 202003N4239), the Ningbo “Technology Innovation 2025” Major Special Project (grant nos. 2018B10015, 2020Z097), the Public Welfare Fund Research Project of Zhejiang Province (No. LGJ22H130001), and the Key Project of Teaching and Research of Ningbo University (grant no. JYXMXZD2021033).
Funding
This work was supported by grants from the Natural Science Foundation of Zhejiang Provincial (grant nos. LY20H130001, LQ21H130001), Ningbo Health Branding Subject Fund (grant no. PPXK2018-02), Ningbo Natural Science Foundation (grant no. 202003N4239), the Ningbo “Technology Innovation 2025” Major Special Project (grant nos. 2018B10015, 2020Z097), the Public Welfare Fund Research Project of Zhejiang Province (No. LGJ22H130001), and the Key Project of Teaching and Research of Ningbo University (grant no. JYXMXZD2021033). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, H., Qu, S., Deng, Y. et al. Application of organoids in otolaryngology: head and neck surgery. Eur Arch Otorhinolaryngol 281, 1643–1649 (2024). https://doi.org/10.1007/s00405-023-08348-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-08348-4