Abstract
Background
Chemotherapy negatively affects gonadal function, often resulting in premature ovarian failure (POF) due to ovarian reserve depletion. Mechanisms of gonadotoxicity, such as primordial follicle overactivation and “burnout”, remain to be established. Ovarian tissue cryopreservation (OTC) before treatment plays an important role in safeguarding fertility.
Methods
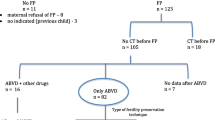
This is a prospective observational study that aims to evaluate the feasibility of OTC after chemotherapeutic treatment initiation. Patients were divided into 2 groups depending on whether they received chemotherapy before the harvesting procedure (Group 1) or not (Group 2). The main outcomes of this study are serum anti-Mullerian hormone (AMH) levels and histological follicular counts on ovarian tissue biopsies.
Results
Between 2012 and 2020, 79 patients underwent OTC at our Hospital. Follicular counts from the ovarian biopsies of 30 post-pubertal patients and respective serum AMH levels were included in the analysis. AMH levels did not significantly differ between the 2 groups (P = 0.70) as well as the number of primordial follicles (P = 0.73). Ovarian biopsies of patients from Group 1 showed a higher number of primary follicles (P = 0.04) and atretic follicles (P = 0.05) with respect to Group 2.
Conclusions
In conclusion, OTC appears to be feasible even after the start of chemotherapeutic treatment, since in treated patients, the main ovarian reserve indicators (number of primordial follicles and serum AMH levels) were not significantly reduced compared to untreated patients. The “burnout” theory of chemotherapeutic damage to the ovary seems to be supported by the higher number of primary follicles found in the ovaries of patients who received chemotherapy before OTC.



Similar content being viewed by others
References
DeVita VT, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68(21):8643–8653
American cancer society cancer treatment & survivorship facts & figures 2019–2021 Atlanta: american cancer society 2019 Available from: https://www.cancer.org/research/cancer-facts-statistics/survivor-facts-figures.html Accessed on 26/02/2020
Meirow D, Nugent D (2001) The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update 7(6):535–543
Liedtke C, Kiesel L (2012) Chemotherapy-induced amenorrhea an update Geburtshilfe und Frauenheilkunde. Geburtshilfe Frauenheilkd 72(9):809–818
Peigné M, Decanter C (2014) Serum AMH level as a marker of acute and long-term effects of chemotherapy on the ovarian follicular content: A systematic review. Reprod Biol Endocrinol 26(12):26
Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E (2007) Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer 43(11):1646–1653
Wallace WHB, Kelsey TW (2010) Human ovarian reserve from conception to the menopause. PLoS ONE 5(1):1–9
Meirow D, Biederman H, Anderson RA, Wallace WHB (2010) Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol 53(4):727–739
Donnez J, Dolmans MM (2017) Fertility preservation in women. N Engl J Med 377(17):1657–1665
Cardozo ER, Thomson AP, Karmon AE, Dickinson KA, Wright DL, Sabatini ME (2015) Ovarian stimulation and in-vitro fertilization outcomes of cancer patients undergoing fertility preservation compared to age matched controls: a 17-year experience. J Assist Reprod Genet 32(4):587–596
Meirow D, Ra’anani H, Shapira MB et al (2016) Transplantations of frozen-thawed ovarian tissue demonstrate high reproductive performance and the need to revise restrictive criteria. Fertil Steril 106(2):467–474
Practice Committee of the American Society for Reproductive Medicine (2019) Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril 112(6):1022–1033
Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE, Wallace WH (2015) Cancer treatment and gonadal function: experimental and established strategies for fertility preservation in children and young adults. Lancet Diabetes Endocrinol 3(7):556–567
Meirow D, Levron J, Eldar-Geva T et al (2005) Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med 353(3):318–321
Poirot C, Fortin A, Lacorte JM et al (2019) Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum Reprod 34(6):1083–1094
Bedoschi G, Navarro PA, Oktay K (2016) Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Futur Oncol 12(19):2333–2344
Wynn TA, Ramalingam TR (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7):1028–1040
Abir R, Ben-Haroush A, Felz C et al (2008) Selection of patients before and after anticancer treatment for ovarian cryopreservation. Hum Reprod 23(4):869–877
Oktem O, Oktay K (2007) Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 110(10):2222–2229
Oktem O, Oktay K (2007) A novel ovarian xenografting model to characterize the impact of chemotherapy agents on human primordial follicle reserve. Cancer Res 67(21):10159–10162
Meirow D, Lewis H, Nugent D, Epstein M (1999) Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod 14(7):1903–1907
Hansen KR, Hodnett GM, Knowlton N, Craig LB (2011) Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 95(1):170–175
Sonigo C, Beau I, Grynberg M, Binart N (2019) AMH prevents primordial ovarian follicle loss and fertility alteration in cyclophosphamide-treated mice. FASEB J 33(1):1278–1287
Roness H, Kashi O, Meirow D (2016) Prevention of chemotherapy-induced ovarian damage. Fertil Steril 105(1):20–29
Kalich-Philosoph L, Roness H, Carmely A et al (2013) Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci Transl Med 5(185):185ra62
Chang EM, Lim E, Yoon S et al (2015) Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3α pathway which leads to loss of ovarian reserve in mice. PLoS ONE 10(12):1–16
Wang Y, Liu M, Johnson SB et al (2019) Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation. Toxicol Appl Pharmacol 381(April):114714
Zhou L, Xie Y, Li S et al (2017) Rapamycin Prevents cyclophosphamide-induced over-activation of primordial follicle pool through PI3K/Akt/mTOR signaling pathway in vivo. J Ovarian Res 10(1):1–11
Lande Y, Fisch B, Tsur A et al (2017) Short-term exposure of human ovarian follicles to cyclophosphamide metabolites seems to promote follicular activation in vitro. Reprod Biomed Online 34(1):104–114
Spears N, Lopes F, Stefansdottir A et al (2019) Ovarian damage from chemotherapy and current approaches to its protection. Hum Reprod Update 25(6):673–693
Donnez J, Dolmans MM (2015) Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice. J Assist Reprod Genet 32(8):1167–1170
Jensen AK, Macklon KT, Fedder J, Ernst E, Humaidan P, Andersen CY (2016) 86 successful births and 9 ongoing pregnancies worldwide in women transplanted with frozen-thawed ovarian tissue: focus on birth and perinatal outcome in 40 of these children. J Assist Reprod Genet 34(3):325–336
Funding
The authors have not received any funding to support this study.
Author information
Authors and Affiliations
Contributions
RC’s roles included conceptualization, formal analysis and manuscript drafting; GM. contributed to conceptualization, critical discussion of data and manuscript review; GT. and LC performed data curation and formal analysis, and contributed to manuscript drafting; LP, EP, AB, SF and MC contributed to critical discussion of data and manuscript review. All authors read and approved the final version of the manuscript. The present work was performed by RC in partial fulfillment of the requirements for obtaining the PhD degree at Vita-Salute San Raffaele University, Milano, Italy.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest relevant to the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cioffi, R., Cervini, L., Taccagni, G. et al. A prospective, observational study of chemotherapy-induced ovarian damage on follicular reserve and maturation. Arch Gynecol Obstet 306, 1723–1729 (2022). https://doi.org/10.1007/s00404-022-06692-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06692-0