Abstract
Purpose
The aim of this study is to investigate the placental expression of VEGF and CD31 in pregnancies complicated by gestational diabetes (GDM) and the influence of pregestational BMI and gestational weight gain (GWG) on this expression.
Methods
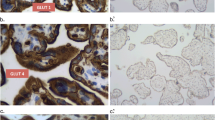
We prospectively enrolled pregnant women with diagnosis of GDM and healthy controls who delivered in our Center between December 2016 and May 2017. Patients were grouped according to the presence of GDM and we compared pregnancy characteristics, placental VEGF and CD31 expression between the cases and controls. Immunochemistry analysis was performed to assess biomarkers positivity. Positivity of biomarkers was assessed in a dichotomic fashion with positivity set at 5% for VEGF and 1% for CD31.
Results
39 patients matched inclusion criteria, 29 (74.3%) women with GDM and 10 (25.7%) healthy controls. Immunochemistry analysis showed that VEGF was more expressed in placentas from women with GDM compared to controls (21/29, 72.4% vs 2/10, 20%; p = 0.007), and CD31 was more expressed in placentas from women with GDM compared to controls (6/29, 20.7% vs 0/10, 0%; risk difference 0.2). VEGF positivity was associated with the presence of GDM (aOR 22.02, 95% CI 1.13–428.08, p = 0.04), pregestational BMI (aOR 1.53, 1.00–2.34, p = 0.05) and GWG (aOR 1.47, 95% CI 1.03–2.11, p = 0.03). CD31 positivity was associated with the pregestational BMI (aOR 1.47, 95% CI 1.00–2.17, p = 0.05) and with the gestational weight gain (aOR 1.32, 95% CI 1.01–1.72, p = 0.04).
Conclusion
Pregnancies complicated by GDM are characterized by increased placental expression of VEGF and CD31, and the expression of these markers is also independently associated to maternal increased pregestational BMI and GWG, defining the concept of “placental diabesity”.

Similar content being viewed by others
References
International Diabetes Federation (2013) IDF Atlas, 6th edn. International Diabetes Federation, Brussels
Hod M, Kapur A, Sacks DA, Hadar E, Agarwal M, Di Renzo GC, Cabero Roura L, McIntyre HD, Morris JL, Divakar H (2015) The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 131(Suppl 3):S173-211. https://doi.org/10.1016/S0020-7292(15)30033-3 (PMID: 26433807)
Dsouza R, Horyn I, Pavalagantharajah S, Zaffar N, Jacob CE (2019) Maternal body mass index and pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol MFM 1(4):100041. https://doi.org/10.1016/j.ajogmf.2019.100041 (Epub 2019 Aug 30. PMID: 33345836)
Bellos I, Fitrou G, Pergialiotis V, Perrea DN, Daskalakis G (2019) Serum levels of adipokines in gestational diabetes: a systematic review. J Endocrinol Invest 42(6):621–631. https://doi.org/10.1007/s40618-018-0973-2 (Epub 2018 Nov 3. PMID: 30392100)
Champion ML, Harper LM (2020) Gestational weight gain: update on outcomes and interventions. Curr Diab Rep 20(3):11. https://doi.org/10.1007/s11892-020-1296-1
Licht P, Russu V, Lehmeyer S, Wissentheit T, Siebzehnrübl E, Wildt L (2003) Cycle dependency of intrauterine vascular endothelial growth factor levels is correlated with decidualization and corpus luteum function. Fertil Steril 80(5):1228–1233. https://doi.org/10.1016/s0015-0282(03)02165-4 (PMID: 14607580)
Goodman C, Jeyendran RS, Coulam CB (2008) Vascular endothelial growth factor gene polymorphism and implantation failure. Reprod Biomed Online 16(5):720–723. https://doi.org/10.1016/s1472-6483(10)60487-7 (PMID: 18492378)
Zeng H, Hu L, Xie H et al (2021) Polymorphisms of vascular endothelial growth factor and recurrent implantation failure: a systematic review and meta-analysis. Arch Gynecol Obstet 304:297–307. https://doi.org/10.1007/s00404-021-06072-0
Augustine G, Pulikkathodi M, Renjith S, Jithesh TK (2016) A study of placental histological changes in gestational diabetes mellitus on account of fetal hypoxia. Int J Med Sci Public Heal 5:2457. https://doi.org/10.5455/ijmsph.2016.29042016494
Madhuri K, Jyothi I (2017) A study on placental morphology in gesatational diabetes. J Evid Based Med Healthc 4:71–75. https://doi.org/10.18410/jebmh/2017/14
Mitanchez D, Yzydorczyk C, Siddeek B et al (2015) The offspring of the diabetic mother—short- and long-term implications. Best Pract Res Clin Obstet Gynaecol 29:256–269
Magee TR, Ross MG, Wedekind L, Desai M, Kjos S, Belkacemi L (2014) Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J Diabetes Complications 28(4):448–459. https://doi.org/10.1016/j.jdiacomp.2014.03.010 (Epub 2014 Mar 24. PMID: 24768206; PMCID: PMC4166519)
Li HP, Chen X, Li MQ (2013) Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol 6(4):650–659 (Epub 2013 Mar 15. PMID: 23573311; PMCID: PMC3606854)
Woodfin A, Voisin MB, Nourshargh S (2007) PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 27(12):2514–2523. https://doi.org/10.1161/ATVBAHA.107.151456 (Epub 2007 Sep 13. PMID: 17872453)
Meng Q, Shao L, Luo X, Mu Y, Xu W, Gao L, Xu H, Cui Y (2016) Expressions of VEGF-A and VEGFR-2 in placentae from GDM pregnancies. Reprod Biol Endocrinol 14(1):61. https://doi.org/10.1186/s12958-016-0191-8 (PMID: 27645229; PMCID: PMC5029036)
Troncoso F, Acurio J, Herlitz K, Aguayo C, Bertoglia P, Guzman-Gutierrez E, Loyola M, Gonzalez M, Rezgaoui M, Desoye G, Escudero C (2017) Gestational diabetes mellitus is associated with increased pro-migratory activation of vascular endothelial growth factor receptor 2 and reduced expression of vascular endothelial growth factor receptor 1. PLoS ONE 12(8):e0182509. https://doi.org/10.1371/journal.pone.0182509 (PMID: 28817576; PMCID: PMC5560693)
Zhou J, Ni X, Huang X, Yao J, He Q, Wang K, Duan T (2016) Potential role of hyperglycemia in fetoplacental endothelial dysfunction in gestational Diabetes mellitus. Cell Physiol Biochem 39(4):1317–1328. https://doi.org/10.1159/000447836 (Epub 2016 Sep 8. PMID: 27606810)
Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline (2014) Diabetes Research and Clinical Practice. 103(3):341–363. https://doi.org/10.1016/j.diabres.2013.10.012 (PMID: 24847517)
Wang Q, Sun L, Yan J, Wang S, Zhang J, Zheng X (2017) Expression of vascular endothelial growth factor and caspase-3 in mucinous breast carcinoma and infiltrating ductal carcinoma-not otherwise specified, and the correlation with disease-free survival. Oncol Lett 14:4890–4896
Bursac Z, Gauss CH, Williams DK et al (2008) Purposeful selection of variables in logistic regression. Source Code Biol Med 3:17. https://doi.org/10.1186/1751-0473-3-17
Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM (1998) Longitudinal changes in maternal serum leptin concentrations, body composition, and resting metabolic rate in pregnancy. Am J Obstet Gynecol 178(5):1010–1015. https://doi.org/10.1016/s0002-9378(98)70540-x (PMID: 9609576)
Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL (1993) Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132(2):879–887. https://doi.org/10.1210/endo.132.2.8425500 (PMID: 8425500)
Rieck S, Kaestner KH (2010) Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab 21(3):151–158. https://doi.org/10.1016/j.tem.2009.11.001 (Epub 2009 Dec 16. PMID: 20015659; PMCID: PMC3627215)
Ashcroft FM, Rohm M, Clark A, Brereton MF (2017) Is type 2 diabetes a glycogen storage disease of pancreatic β cells? Cell Metab 26(1):17–23. https://doi.org/10.1016/j.cmet.2017.05.014 (PMID: 28683284; PMCID: PMC5890904)
Khambule L, George JA (2019) The Role of inflammation in the development of GDM and the use of markers of inflammation in GDM screening. Adv Exp Med Biol 1134:217–242. https://doi.org/10.1007/978-3-030-12668-1_12 (PMID: 30919340)
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N (2014) Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105(2):141–150. https://doi.org/10.1016/j.diabres.2014.04.006 (Epub 2014 Apr 13. PMID: 24798950)
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. https://doi.org/10.1101/cshperspect.a001651 (PMID: 20457564; PMCID: PMC2882124)
Stephens JM, Lee J, Pilch PF (1997) Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem 272(2):971–976. https://doi.org/10.1074/jbc.272.2.971 (PMID: 8995390)
Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM (1994) Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA 91(11):4854–4858. https://doi.org/10.1073/pnas.91.11.4854 (PMID: 8197147; PMCID: PMC43887)
Kinalski M, Telejko B, Kuźmicki M, Kretowski A, Kinalska I (2005) Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res 37(7):450–454. https://doi.org/10.1055/s-2005-870238 (PMID: 16034719)
Huynh J, Dawson D, Roberts D, Bentley-Lewis R (2015) A systematic review of placental pathology in maternal diabetes mellitus. Placenta 36(2):101–114. https://doi.org/10.1016/j.placenta.2014.11.021 (Epub 2014 Dec 5. PMID: 25524060; PMCID: PMC4339292)
Fadini GP, Albiero M, Bonora BM, Avogaro A (2019) Angiogenic abnormalities in diabetes mellitus: mechanistic and clinical aspects. J Clin Endocrinol Metab 104(11):5431–5444. https://doi.org/10.1210/jc.2019-00980 (PMID: 31211371)
Djelmiš J, Desoye G, Ivaniševic M (eds) (2005) Diabetology of pregnancy. Front Diabetes. 17:110–126 https://doi.org/10.1159/000087407
Thangarajah H, Vial IN, Grogan RH, Yao D, Shi Y, Januszyk M, Galiano RD, Chang EI, Galvez MG, Glotzbach JP, Wong VW, Brownlee M, Gurtner GC (2010) HIF-1a dysfunction in diabetes. Cell Cycle 9(1):75–79
Venneri MA, Barbagallo F, Fiore D, De Gaetano R, Giannetta E, Sbardella E, Pozza C, Campolo F, Naro F, Lenzi A, Isidori AM (2019) PDE5 inhibition stimulates Tie2-expressing monocytes and angiopoietin-1 restoring angiogenic homeostasis in diabetes. J Clin Endocrinol Metab 104(7):2623–2636
Okamoto T, Tanaka S, Stan AC, Koike T, Kase M, Makita Z, Sawa H, Nagashima K (2002) Advanced glycation end products induce angiogenesis in vivo. Microvasc Res 63:186–195
Hellström M, Gerhardt H, Kalén M, Li X, Eriksson U, Wolburg H, Betsholtz C (2001) Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol 153:543–553
Warmke N, Griffin KJ, Cubbon RM (2016) Pericytes in diabetes associated vascular disease. J Diabetes Complications 30(8):1643–1650
Davis LE, Widness JA, Brace RA (2003) Renal and placental secretion of erythropoietin during anemia or hypoxia in the ovine fetus. Am J Obstet Gynecol 189:1764–1770
Benyo DF, Conrad KP (1999) Expression of the erythropoietin receptor by trophoblast cells in the human placenta. Biol Reprod 60:861–870
Kim SY, Sappenfield W, Sharma AJ, Wilson HG, Bish CL, Salihu HM, England LJ (2013) Racial/ethnic differences in the prevalence of gestational diabetes mellitus and maternal overweight and obesity, by nativity, Florida, 2004–2007. Obesity (Silver Spring) 21(1):E33-40. https://doi.org/10.1002/oby.20025 (PMID: 23404915; PMCID: PMC4392762)
Grieger JA, Leemaqz SY, Knight EJ et al (2022) Relative importance of metabolic syndrome components for developing gestational diabetes. Arch Gynecol Obstet 305:995–1002. https://doi.org/10.1007/s00404-021-06279-1
Xi F, Chen H, Chen Q et al (2021) Second-trimester and third-trimester maternal lipid profiles significantly correlated to LGA and macrosomia. Arch Gynecol Obstet 304:885–894. https://doi.org/10.1007/s00404-021-06010-0
Yoles I, Sheiner E, Wainstock T (2021) First pregnancy risk factors and future gestational diabetes mellitus. Arch Gynecol Obstet 304:929–934. https://doi.org/10.1007/s00404-021-06024-8
López-Tinoco C, Roca M, Fernández-Deudero A, García-Valero A, Bugatto F, Aguilar-Diosdado M, Bartha JL (2012) Cytokine profile, metabolic syndrome and cardiovascular disease risk in women with late-onset gestational diabetes mellitus. Cytokine 58(1):14–19. https://doi.org/10.1016/j.cyto.2011.12.004 (Epub 2011 Dec 24. PMID: 22200508)
Richardson AC, Carpenter MW (2007) Inflammatory mediators in gestational diabetes mellitus. Obstet Gynecol Clin North Am 34(2):213–224. https://doi.org/10.1016/j.ogc.2007.04.001 (PMID: 17572268)
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112(12):1796–1808. https://doi.org/10.1172/JCI19246 (PMID: 14679176; PMCID: PMC296995)
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445. https://doi.org/10.1146/annurev-immunol-031210-101322 (PMID: 21219177)
Bergmann K, Sypniewska G (2013) Diabetes as a complication of adipose tissue dysfunction. Is there a role for potential new biomarkers? Clin Chem Lab Med 51(1):177–185. https://doi.org/10.1515/cclm-2012-0490 (PMID: 23241684)
Dubova EA, Pavlov KA, Borovkova EI, Bayramova MA, Makarov IO, Shchegolev AI (2011) Vascular endothelial growth factor and its receptors in the placenta of pregnant women with obesity. Bull Exp Biol Med 151(2):253–258. https://doi.org/10.1007/s10517-011-1302-3 (PMID: 22238763.51)
Yi QY, Deng G, Chen N et al (2016) Metformin inhibits development of diabetic retinopathy through inducing alternative splicing of VEGF-A. Am J Transl Res 8(9):3947–3954
Joe SG, Yoon YH, Choi JA, Koh J-Y (2015) Anti-angiogenic effect of metformin in mouse oxygen-induced retinopathy is mediated by reducing levels of the vascular endothelial growth factor receptor Flk-1. PLoS ONE 10(3):e0119708. https://doi.org/10.1371/journal.pone.0119708
Zhang K, Han ES, Dellinger TH, Lu J, Nam S, Anderson RA, Yim JH, Wen W (2017) Cinnamon extract reduces VEGF expression via suppressing HIF-1α gene expression and inhibits tumor growth in mice. Mol Carcinog 56(2):436–446. https://doi.org/10.1002/mc.22506
Hosni A, El-Twab SA, Abdul-Hamid M, Prinsen E, AbdElgawad H, Abdel-Moneim A, Beemster GTS (2021) Cinnamaldehyde mitigates placental vascular dysfunction of gestational diabetes and protects from the associated fetal hypoxia by modulating placental angiogenesis, metabolic activity and oxidative stress. Pharmacol Res 165:105426. https://doi.org/10.1016/j.phrs.2021.105426
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: AS and DP; methodology: VAD; validation: VA, AL and DP; formal analysis: EDR; investigation: AS; resources: AR and MDL; data curation: LT; writing—original draft preparation: AS; writing—review and editing: DP; visualization: VA; supervision: AL; project administration: AS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sirico, A., Rossi, E.D., Degennaro, V.A. et al. Placental diabesity: placental VEGF and CD31 expression according to pregestational BMI and gestational weight gain in women with gestational diabetes. Arch Gynecol Obstet 307, 1823–1831 (2023). https://doi.org/10.1007/s00404-022-06673-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06673-3