Abstract
Objective
To validate our previous findings of high-level EGFR expression in GCCC using an expanded cohort of specimens and to further examine the molecular and cellular features of this aggressive malignancy to identify potentially actionable therapeutic targets.
Methods
The SEER database was queried to obtain the epidemiological data regarding the current national survival trends for GCCC. Immunohistochemistry (IHC) was used to examine the expression of EGFR, PD-1, and PD-L1. CiberSort analysis was used to analyze a previously published RNA-sequencing dataset obtained from a single patient diagnosed with GCCC.
Results
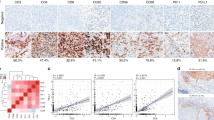
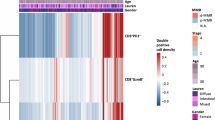
In comparison to squamous cell carcinomas and adenocarcinoma/adenosquamous carcinomas, GCCC was observed in younger patients (p < 0.001) and demonstrated inferior survival (p < 0.001). All (100%) of the specimens (8/8) exhibited immunoreactivity when stained for CD3ε (T-cell marker), EGFR, PD-1, and PD-L1 whereas CTLA4 expression was not detected. Analysis of RNA-sequencing data revealed that cetuximab and erlotinib altered the chemokine profile, lymphocyte abundance, and expression of inhibitory immune checkpoints in a single patient when combined with cytotoxic chemotherapy in a single patient.
Conclusions
The data from this descriptive study suggests that immune checkpoint blockade, whether single agent or in combination, may be a suitable therapeutic option for a disease for which targeted approaches do not currently exist.







Similar content being viewed by others
Data availability
Data and material available for review upon request.
References
Hopkins MP, Morley GW (2004) Glassy cell adenocarcinoma of the uterine cervix. Am J Obstet Gynecol 190(1):67–70. https://doi.org/10.1016/s0002-9378(03)00928-1 (Epub 2004/01/30, PubMed PMID: 14749637)
Zolciak-Siwinska A, Jonska-Gmyrek J (2014) Glassy cell carcinoma of the cervix: a literature review. Eur J Obstet Gynecol Reprod Biol 179:232–235. https://doi.org/10.1016/j.ejogrb.2014.03.035 (Epub 2014/05/06, PubMed PMID: 24792540)
Guitarte C, Alagkiozidis I, Mize B, Stevens E, Salame G, Lee YC (2014) Glassy cell carcinoma of the cervix: a systematic review and meta-analysis. Gynecol Oncol 133(2):186–191. https://doi.org/10.1016/j.ygyno.2014.01.048 (Epub 2014/02/08, PubMed PMID: 24503463)
Jung YY, Nahm JH, Kim HS (2016) Cytomorphological characteristics of glassy cell carcinoma of the uterine cervix: histopathological correlation and human papillomavirus genotyping. Oncotarget 7(45):74152–74161. https://doi.org/10.18632/oncotarget.12361 (Epub 2016/10/07, PubMed PMID: 27708230; PubMed Central PMCID: PMCPMC5342042)
Littman P, Clement PB, Henriksen B, Wang CC, Robboy SJ, Taft PD et al (1976) Glassy cell carcinoma of the cervix. Cancer 37(5):2238–2246. https://doi.org/10.1002/1097-0142(197605)37:5%3c2238::aid-cncr2820370513%3e3.0.co;2-d (Epub 1976/05/01, PubMed PMID: 1260713)
Kuroda H, Toyozumi Y, Masuda T, Ougida T, Hanami K, Kyoko K et al (2006) Glassy cell carcinoma of the cervix: cytologic features and expression of estrogen receptor, progesterone receptor and Her2/neu protein. Acta Cytol 50(4):418–422. https://doi.org/10.1159/000325985 (Epub 2006/08/12, PubMed PMID: 16901007)
Chung JH, Koh JS, Lee SS, Cho KJ (2000) Glassy cell carcinoma of the uterine cervix. Cytologic features and expression of estrogen and progesterone receptors. Acta Cytol 44(4):551–556. https://doi.org/10.1159/000328529 (Epub 2000/08/10, PubMed PMID: 10934948)
Yoon N, Kim JY, Kim HS (2016) Clinical outcomes of advanced-stage glassy cell carcinoma of the uterine cervix: a need for reappraisal. Oncotarget 7(48):78448–78454. https://doi.org/10.18632/oncotarget.12905 (Epub 2016/10/30, PubMed PMID: 27793022; PubMed Central PMCID: PMCPMC5346652)
Gray HJ, Garcia R, Tamimi HK, Koh WJ, Goff BA, Greer BE et al (2002) Glassy cell carcinoma of the cervix revisited. Gynecol Oncol 85(2):274–277. https://doi.org/10.1006/gyno.2001.6523 (Epub 2002/04/26, PubMed PMID: 11972387)
McEachron TA, Sender LS, Zabokrtsky KB, Kaltenecker B, Holmes WN, Cherni I et al (2016) Molecular genetic profiling of adolescent glassy cell carcinoma of the cervix reveals targetable EGFR amplification with potential therapeutic implications. J Adolesc Young Adult Oncol 5(3):297–302. https://doi.org/10.1089/jayao.2015.0068 (Epub 2016/03/15, PubMed PMID: 26974246)
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y et al (2015) Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 12(5):453–457. https://doi.org/10.1038/nmeth.3337 (Epub 2015/03/31, PubMed PMID: 25822800; PubMed Central PMCID: PMCPMC4739640.)
Lin A, Wei T, Meng H, Luo P, Zhang J (2019) Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer 18(1):139. https://doi.org/10.1186/s12943-019-1062-7 (Epub 2019/09/19, PubMed PMID: 31526368; PubMed Central PMCID: PMCPMC6745797)
Jia Y, Li X, Jiang T, Zhao S, Zhao C, Zhang L et al (2019) EGFR-targeted therapy alters the tumor microenvironment in EGFR-driven lung tumors: implications for combination therapies. Int J Cancer 145(5):1432–1444. https://doi.org/10.1002/ijc.32191 (Epub 2019/02/21, PubMed PMID: 30784054)
Chung HC, Ros W, Delord JP, Perets R, Italiano A, Shapira-Frommer R et al (2019) Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 37(17):1470–1478. https://doi.org/10.1200/jco.18.01265 (Epub 2019/04/04, PubMed PMID: 30943124)
Teng MW, Ngiow SF, Ribas A, Smyth MJ (2015) Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 75(11):2139–2145. https://doi.org/10.1158/0008-5472.can-15-0255 (Epub 2015/05/16, PubMed PMID: 25977340; PubMed Central PMCID: PMCPMC4452411)
Sznol M, Chen L (2013) Antagonist antibodies to PD-1 and B7–H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 19(5):1021–1034. https://doi.org/10.1158/1078-0432.ccr-12-2063 (Epub 2013/03/06, PubMed PMID: 23460533; PubMed Central PMCID: PMCPMC3702373)
Angell H, Galon J (2013) From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 25(2):261–267. https://doi.org/10.1016/j.coi.2013.03.004 (Epub 2013/04/13, PubMed PMID: 23579076)
Li Q, Cheng X, Ji J, Zhang J, Zhou X (2014) Gene amplification of EGFR and its clinical significance in various cervical (lesions) lesions using cytology and FISH. Int J Clin Exp Pathol 7(5):2477–2483 (Epub 2014/06/27, PubMed PMID: 24966959; PubMed Central PMCID: PMCPMC4069959)
Li Q, Tang Y, Cheng X, Ji J, Zhang J, Zhou X (2014) EGFR protein expression and gene amplification in squamous intraepithelial lesions and squamous cell carcinomas of the cervix. Int J Clin Exp Pathol 7(2):733–741 (Epub 2014/02/20, PubMed PMID: 24551297; PubMed Central PMCID: PMCPMC3925921)
Conesa-Zamora P, Torres-Moreno D, Isaac MA, Perez-Guillermo M (2013) Gene amplification and immunohistochemical expression of ERBB2 and EGFR in cervical carcinogenesis. Correlation with cell-cycle markers and HPV presence. Exp Mol Pathol 95(2):151–155. https://doi.org/10.1016/j.yexmp.2013.06.011 (Epub 2013/07/06, PubMed PMID: 23827764)
Bumrungthai S, Munjal K, Nandekar S, Cooper K, Ekalaksananan T, Pientong C et al (2015) Epidermal growth factor receptor pathway mutation and expression profiles in cervical squamous cell carcinoma: therapeutic implications. J Transl Med 13:244. https://doi.org/10.1186/s12967-015-0611-0 (Epub 2015/07/26, PubMed PMID: 26209091; PubMed Central PMCID: PMCPMC4513684)
Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL et al (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3(12):1355–1363. https://doi.org/10.1158/2159-8290.cd-13-0310 (Epub 2013/10/01, PubMed PMID: 24078774; PubMed Central PMCID: PMCPMC3864135)
Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T et al (2014) Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 25(10):1935–1940. https://doi.org/10.1093/annonc/mdu242 (Epub 2014/07/11, PubMed PMID: 25009014)
Thumallapally N, Parylo S, Vennepureddy A, Ibrahim U, Sokoloff A (2018) Synchronous presence of EGFR, ALK driver mutations along with PD L1 overexpression in a resected early stage non-small cell lung cancer: a case report and review of literature. World J Oncol 9(2):50–55. https://doi.org/10.14740/wjon1090e (Epub 2018/05/16, PubMed PMID: 29760833; PubMed Central PMCID: PMCPMC5942208)
Acknowledgements
We thank Jean Richardson for support of the USC Department of Obstetrics & Gynecology Gynecologic Tissue and Fluid Repository and the Norris Comprehensive Cancer Center Translational Pathology Core (NIH P30CA014089).
Funding
Funded by USC Department of Gynecologic Oncology Research Fund.
Author information
Authors and Affiliations
Contributions
EB: principal investigator and manuscript author; TM: senior author, project concept and execution; MR: UMPC principal investigator; MR: University of Colorado Principal Investigator; RB: pathologist at UMPC; MP: pathologist at University of Colorado; SW: pathologist at USC, reviewed all samples for consistency; KM: SEER database analysis and figures; DdS: basic science advisory role; LR: Gyn Onc faculty supervisor; LT: basic science contributor; ES: logistical support.
Corresponding author
Ethics declarations
Conflict of interest
Erin Blake: none; Troy McEachron: none; Malcolm Ross: none; Megan Ross: none; Rohit Bhargava: none; Miriam Post: none; Saloni Walia: none; Koji Matsuo: Chugai (honorarium 2016), Springer (textbook editorial expense 2018), VBL Therapeutics (investigator meeting attendance expense 2019); Diane da Silva: Venn Biosciences (grant researching ovarian cancer biomarkers), Quantum screening (consulting fees); Lynda Roman: Tempus Lab (consultant > 2 years ago, none relevant to submission), Quantgene (consultant, not relevant to submission); Lilibeth Torno: none; Emily Silverstein: none.
Ethical approval
USC Health Sciences Institutional Review Board approved (HS-18-00266). Approval letter available upon request.
Informed consent
Informed consent was not necessary for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
404_2021_6164_MOESM1_ESM.pdf
Supplementary file1 Expression of CD3ε, CD8, PD-L1, PD1, and EGFR in cervical tissue unaffected by disease (PDF 13404 KB)
Rights and permissions
About this article
Cite this article
Blake, E.A., Ross, M.S., Ross, M.E. et al. Immunohistochemical analysis of glassy cell carcinoma of the cervix reveals robust lymphocyte infiltrate and the expression of targetable inhibitory immune checkpoints. Arch Gynecol Obstet 305, 439–447 (2022). https://doi.org/10.1007/s00404-021-06164-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06164-x