Abstract
Introduction
Orlistat possesses anti-tumor capacity by inducing apoptosis in ovarian cancer cells. However, the mechanism is not clearly understood. Emerging evidence indicates the overlaps between autophagy and apoptosis. In this study, we have investigated the role of autophagy in orlistat-induced apoptosis in ovarian cancer (OC) cells.
Methods
The effect of orlistat on apoptosis was evaluated in SKOV3 and A2780 cell lines by MTT and TUNEL assay. The formations of autophagosomes were observed by acridine orange and GFP-LC3 fluorescence. In addition, conversions of LC3-I to LC3-II were analyzed by western blot, as well as other autophagy-related proteins. 3-Methyladenine (3-MA) was used as an autophagy inhibitor in combined treatment with orlistat. Western blot was further conducted to investigate the molecular mechanisms of orlistat-affected apoptosis and autophagy on protein level.
Results
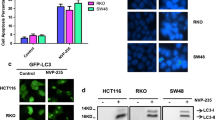
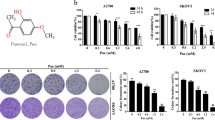
The proliferation activities of OC cells were inhibited by orlistat in a dose-dependent manner. The expressions of cleaved-caspase 3 and 9 in orlistat-treated cells were increasing, which suggested that orlistat-induced apoptosis was caspase-dependent. At the same time, the average number of GFP-LC3 dots per cell was increased after 48 h of orlistat treatment. The expression levels of LC3-II were significantly up-regulated, as well as other autophagy-related proteins such as Vsp34, Atg7 and UVRAG. These results suggested orlistat-induced autophagy flux, which was further found involved in inhibiting the Akt/mTOR/p70S6K signaling pathway. However, combined treatment of orlistat and 3-MA significantly suppressed the cell viability, which indicated a pro-survival role of autophagy in OC cells.
Conclusion
We suggested that orlistat had anti-cancer effect in OC cells. In addition, autophagy played a pro-survival role, suppressing which the orlistat-induced anti-cancer effect would be more significant.





Similar content being viewed by others
References
White KL, Schildkraut JM, Palmieri RT et al (2012) Ovarian cancer risk associated with inherited inflammation-related variants. Cancer Res 72(5):1064–1069
Maier T, Leibundgut M, Boehringer D et al (2010) Structure and function of eukaryotic fatty acid synthases. Q Rev Biophys 43:373–422
Gelebart P, Zak Z, Anand M et al (2012) Blockade of fatty acid synthase triggers significant apoptosis in mantle cell lymphoma. PLoS One 7(4):e33738. https://doi.org/10.1371/journal.pone.0033738
Puig T, Aguilar H, Cufi S et al (2011) A novel inhibitor of fatty acid synthase shows activity against HER2+ breast cancer xenografts and is active in anti-HER2 drug-resistant cell lines. Breast Cancer Res 13(6):R131. https://doi.org/10.1186/bcr3077
Zaytseva YY, Rychahou PG, Gulhati P et al (2012) Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res 72(6):1504–1517. https://doi.org/10.1158/0008-5472.can-11-4057
Di Vizio D, Sotgia F, Williams TM et al (2007) Caveolin-1 is required for the upregulation of fatty acid synthase (FASN), a tumor promoter, during prostate cancer progression. Cancer Biol Ther 6:1263–1268
Gansler TS, Hardman W 3rd, Hunt DA, Schaffel S, Hennigar RA (1997) Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol 28:686–692
Huang HQ, Tang J, Zhou ST et al (2012) Orlistat, a novel potent antitumor agent for ovarian cancer: proteomic analysis of ovarian cancer cells treated with Orlistat. Int J Oncol 41(2):523–532. https://doi.org/10.3892/ijo.2012.1465
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Bossy-Wetzel E, Newmeyer DD, Green DR (1998) Mitochondrial cytochrome c release in apoptosis occurs upstream of DEVD-specific caspase activation and independently of mitochondrial transmembrane depolarization. EMBO J 17(1):37–49. https://doi.org/10.1093/emboj/17.1.37
Porter AG, Janicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Li P, Nijhawan D, Budihardjo I et al (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91(4):479–489
Pattingre S, Tassa A, Qu X et al (2005) Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 122(6):927–939. https://doi.org/10.1016/j.cell.2005.07.002
Mujumdar N, Saluja AK (2010) Autophagy in pancreatic cancer: an emerging mechanism of cell death. Autophagy 6:997–998
Yu L, Alva A, Su H et al (2004) Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science 304(5676):1500–1502. https://doi.org/10.1126/science.1096645
Zeng X, Kinsella TJ (2008) Mammalian target of rapamycin and S6 kinase 1 positively regulate 6-thioguanine-induced autophagy. Cancer Res 68(7):2384–2390. https://doi.org/10.1158/0008-5472.can-07-6163
Paglin S, Hollister T, Delohery T et al (2001) A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res 61:439–444
Rosenfeldt MT, Ryan KM (2011) The multiple roles of autophagy in cancer. Carcinogenesis 32:955–963
Jaber N, Dou Z, Chen JS et al (2012) Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci USA 109(6):2003–2008. https://doi.org/10.1073/pnas.1112848109
Zhao Z, Ni D, Ghozalli I, Pirooz SD et al (2012) UVRAG: at the crossroad of autophagy and genomic stability. Autophagy 8:1392–1393
Codogno P, Meijer AJ (2005) Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ 12(Suppl 2):1509–1518. https://doi.org/10.1038/sj.cdd.4401751
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100:57–70
Schuhr CA, Eisenreich W, Goese M et al (2002) Biosynthetic precursors of the lipase inhibitor lipstatin. J Org Chem 67:2257–2262
Chuang HY, Chang YF, Hwang JJ (2011) Antitumor effect of orlistat, a fatty acid synthase inhibitor, is via activation of caspase-3 on human colorectal carcinoma-bearing animal. Biomed Pharmacother 65(4):286–292. https://doi.org/10.1016/j.biopha.2011.02.016
Fujiwara J, Sowa Y, Horinaka M et al (2011) The anti-obesity drug orlistat promotes sensitivity to TRAIL by two different pathways in hormone-refractory prostate cancer cells. Int J Oncol 40(5):1483–1491. https://doi.org/10.3892/ijo.2012.1353
Seguin F, Carvalho MA, Bastos DC et al (2012) The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br J Cancer. https://doi.org/10.1038/bjc.2012.355
Mizushima N, Levine B, Cuervo AM et al (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Hoyer-Hansen M, Jaattela M (2008) Autophagy: an emerging target for cancer therapy. Autophagy 4(5):574–580
Kondo Y, Kanzawa T, Sawaya R et al (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5(9):726–734. https://doi.org/10.1038/nrc1692
Kanzawa T, Kondo Y, Ito H, Kondo S et al (2003) Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res 63(9):2103–2108
Chen YJ, Huang WP, Yang YC et al (2009) Platonin induces autophagy-associated cell death in human leukemia cells. Autophagy 5(2):173–183
Thorburn A (2008) Apoptosis and autophagy: regulatory connections between two supposedly different processes. Apoptosis 13(1):1–9. https://doi.org/10.1007/s10495-007-0154-9
Funding
This work was supported by the key research and development program of Sichuan Province (Grant No. 2017SZ0002).
Author information
Authors and Affiliations
Contributions
HLP and QW contributed equally to this work. HLP and QW conducted the experiment and wrote the original draft. XZ assisted with study design, methodology design, and reviewed and edited the original draft. XRQ helped conduct the experiment. XW helped in analysis of data and preparation of tables and figures. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Peng, H., Wang, Q., Qi, X. et al. Orlistat induces apoptosis and protective autophagy in ovarian cancer cells: involvement of Akt-mTOR-mediated signaling pathway. Arch Gynecol Obstet 298, 597–605 (2018). https://doi.org/10.1007/s00404-018-4841-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4841-2