Abstract
Objective
To evaluate the effect of Diane-35, alone or in combination with orlistat or metformin, on androgen and body fat percentage parameters in Chinese overweight and obese polycystic ovary syndrome (PCOS) patients with insulin resistance.
Methods
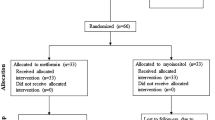
A total of 240 PCOS women were randomly allocated to receive Diane-35 alone (D group), Diane-35 plus orlistat (DO group), Diane-35 plus metformin (DM group), or Diane-35 plus orlistat plus metformin (DOM group). Serum TT, DHEA-S, androstenedione, SHBG, FT, FAI, body fat, and body fat percentage were assessed at baseline and after 12 weeks of treatment.
Results
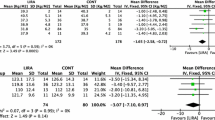
Significant changes in serum TT, SHBG, and FAI were observed in all treatment groups compared with baseline. DHEA-S and androstenedione significantly decreased in the DO, DM, and DOM groups after treatment. FT only significantly decreased in the DOM group. Body fat and body fat percentage significantly decreased in the DO and DOM groups. Compared with the D group, DHEA-S significantly decreased in the DO, DM, and DOM groups (F = 4.081, p = 0.008); SHBG significantly increased in the DOM group (F = 3.019, p = 0.031); and FAI significantly decreased in the DO group (χ2 = 12.578, p = 0.006). There were significant differences between groups in body fat percentage (χ2 = 23.590, p < 0.001). Side-effects were less with orlistat than metformin.
Conclusions
Diane-35 in combination with orlistat or metformin is more effective in reducing androgen than Diane-35 alone. Orlistat is more effective in reducing body fat percentage than metformin. In addition, orlistat has mild side-effects and is better tolerated compared with metformin.


Similar content being viewed by others
References
Goodman NF, Cobin RH, Futerweit W et al (2015) American association of clinical endocrinologists, American college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—PART 2. Endoc Pract 21:1415–1426
Lizneva D, Suturina L, Walker W et al (2016) Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 106:6–15
Ali AT (2015) Polycystic ovary syndrome and metabolic syndrome. Ceska Gynekol 80:279–289
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25
Royal College of Obstetricians and Gynecologists (2014) Polycystic ovary syndrome, long-term consequences (Green-top Guideline No. 33). https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg33/. Accessed 05 Nov 2014
Conway G, Dewailly D, Diamanti-Kandarakis E et al (2014) The polycystic ovary syndrome: a position statement from the European society of endocrinology. Eur J Endocrinol 171:1–29
Buzney E, Sheu J, Buzney E et al (2014) Polycystic ovary syndrome: a review for dermatologists, Part II. Treatment. J Am Acad Dermatol 71:859.e1–859.e15
Ruan X, Kubba A, Aguilar A et al (2017) Use of cyproterone acetate/ethinylestradiol in polycystic ovary syndrome: rationale and practical aspects. Eur J Contracept Reprod Health Care 22:183–190
Williams T, Mortada R, Porter S (2016) Diagnosis and treatment of polycystic ovary syndrome. Am Fam Physician 94:106–113
Wang YW, He SJ, Feng X et al (2017) Metformin: a review of its potential indications. Drug Des Devel Ther 11:2421–2429
Ladson G, Dodson WC, Sweet SD et al (2011) The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril 95:1059–1066
Kumar P, Arora S (2014) Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J Hum Reprod Sci 7:255–261
Alqahtani S, Qosa H, Primeaux B et al (2015) Orlistat limits cholesterol intestinal absorption by Niemann-pick C1-like 1 (NPC1L1) inhibition. Eur J Pharmacol 762:263–269
Ahn SM, Kim H, Ji E et al (2014) The effect of orlistat on weight reduction in obese and overweight Korean patients. Arch Pharm Res 37:512–519
Wu H, Ruan X, Jin J et al (2015) Metabolic profile of Diane-35 versus Diane-35 plus metformin in Chinese PCOS women under standardized life-style changes. Gynecol Endocrinol 31:548–551
Yang YM, Choi EJ (2015) Efficacy and safety of metformin or oral contraceptives, or both in polycystic ovary syndrome. Ther Cin Risk Manag 11:1345–1353
Vilmann LS, Thisted E, Baker JL et al (2012) Development of obesity and polycystic ovary syndrome in adolescents. Horm Res Paediatr 78:269–278
Naderpoor N, Shorakae S, Joham A et al (2015) Obesity and polycystic ovary syndrome. Minerva Endocrinol 40:37–51
Baptiste CG, Battista MC, Trottier A et al (2010) Insulin and hyperandrogenism in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol 122:42–52
Tosi F, Bonora E, Moghetti P (2017) Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod 13:1–7
Nestler JE, Powers LP, Matt DW et al (1991) A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 72:83–89
Feng W, Jia YY, Zhang DY et al (2016) Management of polycystic ovarian syndrome with Diane-35 or Diane-35 plus metformin. Gynecol Endocrinol 32:147–150
Panidis D, Tziomalos K, Papadakis E et al (2014) The role of orlistat in combination with lifestyle changes in the management of overweight and obese patients with polycystic ovary syndrome. Clin Endocrinol (Oxf) 80:432–438
Gallo MF, Lopez LM, Grimes DA et al (2014) Combination contraceptives: effects on weight. Cochrane Database Syst Rev 29:CD003987
Glintborg D, Altinok ML, Mumm H et al (2014) Body composition is improved during 12 months’ treatment with metformin alone or combined with oral contraceptives compared with treatment with oral contraceptives in polycystic ovary syndrome. J Clin Endocrinol Metab 99:2584–2591
de Bastos M, Stegeman BH, Rosendaal FR et al (2014) Combined oral contraceptives: venous thrombosis. Cochrane Database Syst Rev 3:CD010813
Sandset PM (2014) Combined oral contraceptives increase risk of venous thrombosis according to oestrogen dose and type of progestogen. Evid Based Med 19:194
Okoroh EM, Hooper C, Atrash HK et al (2012) Is polycystic ovary syndrome anther risk factor for venous thromboembolism? United States 2003–2008. Am J Obstet Gynecol 207(377):e1–e8
Bird ST, Hartzema AG, Brophy JM et al (2013) Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. CMAJ 185:E115–E120
Acknowledgements
The authors especially thank Prof. Xingming Li for his assistance in statistical analysis of the data.
Author information
Authors and Affiliations
Contributions
XR: Project development, manuscript writing; JS: data collection, data analysis; MG: data collection; LW: data collection; HW: data collection; AOM: manuscript editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by Capital Characteristic Clinic Project of China (Z161100000516143); Beijing Capital Foundation for Medical Science Development and Research (2016-2-2113); Clinical Technique Innovation Project of Beijing Municipal Administration of Hospitals (XMLX201710); Beijing Municipality Health Technology High-level Talent (2014-2-016); Foreign technical and administrative talent introduction project in 2017, State Administration of Foreign Experts Affairs, the P. R. of China (20171100004).
Rights and permissions
About this article
Cite this article
Ruan, X., Song, J., Gu, M. et al. Effect of Diane-35, alone or in combination with orlistat or metformin in Chinese polycystic ovary syndrome patients. Arch Gynecol Obstet 297, 1557–1563 (2018). https://doi.org/10.1007/s00404-018-4762-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-018-4762-0