Abstract
Objective
To assess the efficacy, safety and acceptability of cyclic medroxyprogesterone acetate (MPA) compared to continuous MPA for treatment of endometrial hyperplasia (EH) without atypia.
Materials and methods
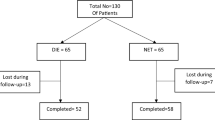
A prospective observational study conducted on premenopausal women with EH without atypia (n = 80) who were randomly assigned into two groups; 40 patients received cyclic 15 mg MPA and 40 patients received continuous 15 mg MPA. Follow-up endometrial sampling was done after 6 months. Primary outcome measure was regression of hyperplasia. Secondary outcome measures include side effects and patient acceptability.
Results
There was no significant difference between the two groups regarding regression of endometrial hyperplasia (90 % in the cyclic MPA group in comparison to 82.5 % in the continuous MPA group with p value >0.05). There was a significant higher women suffering from nausea, acne and menstrual changes in the continuous MPA group (p value <0.05). Cyclic MPA regimen was more acceptable to the patients in comparison to continuous MPA intake.
Conclusions
Cyclic MPA regimen seems a safer and more acceptable therapy in comparison to continuous MPA regimen in patients with endometrial hyperplasia without atypia. Larger studies are warranted to confirm these results.

Similar content being viewed by others
References
Epplein M, Reed SD, Voigt LF, Newton KM, Holt VL, Weiss NS (2009) Endometrial hyperplasia risk in relation to recent use of oral contraceptives and hormone therapy. Ann Epidemiol 19:1–7
Clark TJ, Neelakantan D, Gupta JK (2006) The management of endometrial hyperplasia: an evaluation of current practice. Eur J Obstet Gynecol Reprod Biol 125:259–264
Epplein M, Reed SD, Voigt LF, Newton KM, Holt VL, Weiss NS (2009) Endometrial hyperplasia risk in relation to recent use of oral contraceptives and hormone therapy. Ann Epidemiol 19:1–7
Hammond R, Johnson J (2004) Endometrial hyperplasia. Curr Obstet Gynaecol 14:99–103
American College of Obstetricians and Gynecologists (2005) ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65: management of endometrial cancer. Obstet Gynecol 106:413–425
Buttini MJ, Jordan SJ, Webb PM (2009) The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: an Australian study and systematic review. Aust N Z J Obstet Gynaecol 49(3):316–322
Wang S, Pudney J, Song J, Mor G, Schwartz PE, Zheng W (2003) Mechanisms involved in the evolution of progestin resistance in human endometrial hyperplasia—precursor of endometrial cancer. Gynecol Oncol 88:108–117
Vereide AB, Arnes M, Straume B, Maltau JM, Orbo A (2003) Nuclear morphometric changes and therapy monitoring in patients with endometrial hyperplasia: a study comparing effects of intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol 91:526–533
Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK (2013) LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Hum Reprod 28(11):2966–2971
Gunderson CC, Fader AN, Carson KA, Bristow RE (2012) Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol 125:477–482
Ozdegirmenci O, Kayikcioglu F, Bozkurt U, Akgul MA, Haberal A (2011) Comparison of the efficacy of three progestins in the treatment of simple endometrial hyperplasia without atypia. Gynecol Obstet Invest 72(1):10–14
Orbo A, Vereide A, Arnes M, Pettersen I, Straume B (2014) Levonorgestrel-impregnated intrauterine device as treatment for endometrial hyperplasia: a national multicentre randomised trial. BJOG 121(4):477–486
Chaudhry P, Asselin E (2009) Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr Relat Cancer 16(2):363–380
Goncharenko VM, Beniuk VA, Kalenska OV, Demchenko OM, Spivak MY, Bubnov RV (2013) Predictive diagnosis of endometrial hyperplasia and personalized therapeutic strategy in women of fertile age. EPMA J 4(1):24
Gkrozou F, Dimakopoulos G, Vrekoussis T, Lavasidis L, Koutlas A, Navrozoglou I, Stefos T, Paschopoulos M (2015) Hysteroscopy in women with abnormal uterine bleeding: a meta-analysis on four major endometrial pathologies. Arch Gynecol Obstet 291(6):1347–1354
Acknowledgments
The authors would like to acknowledge the contribution of the residents and nursing staff of the Gynecology ward and members of Pathology department of Menoufia university Hospital. The author would like to thank all the participants for taking out time to share in this study. I also wish to thank Dr. Mohamed Rezk for his logistic support.
Conflict of interest
The author reports no declarations of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emarh, M. Cyclic versus continuous medroxyprogesterone acetate for treatment of endometrial hyperplasia without atypia: a 2-year observational study. Arch Gynecol Obstet 292, 1339–1343 (2015). https://doi.org/10.1007/s00404-015-3749-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3749-3